2025
| Picture |
|---|
| Abstract |
|---|
|
From essentials to applications, the new Handbook of GC-MS: Fundamentals and Applications is a comprehensive reference and training compendium on the popular and evolving technique of GC-MS (gas chromatography/mass spectrometry), guiding readers through the most used sample preparation methods for GC-MS and method development, with many practical indications supporting the design of optimized analyses, and providing practical approaches to data processing, compound identification, and quantification. The text details both a solid background and principles of operation, as well as a broad range of current real-life examples taken from laboratories in environmental, food, pharmaceutical, and forensic analysis. It also features a glossary of more than 300 terms, and a comprehensive substance index that facilitates finding a specific application. This timely Fourth Edition covers the latest developments in automated sample preparation techniques and instrumentation, also with the focus on Green Analytical Chemistry. This comprehensive handbook presents GC-MS applications in various fields, with coverage of the well-known QuEChERS pesticide extraction, solid phase extraction and solid phase microextraction, static and dynamic headspace analysis, liquid/liquid extraction, outgassing, and thermal desorption, including pyrolysis. Single and triple quadrupole, Orbitrap, time-of-flight, magnetic sector, ion mobility and isotope ratio MS are discussed with their advantages and limitations. Sample topics covered in Handbook of GC-MS: Fundamentals and Applications include: • Sample inlet systems for hot needle, liquid band injection with large volume and LC-GC application, carrier gas saving, choice of columns, septa and injection port liners. • Optimization of the GC method with carrier gas flow, effect of oven temperature ramp rates, fast GC, and multi-dimensional gas chromatography. • Ionization processes, electron and chemical ionization, resolution power in mass spectrometry, reading and interpreting mass spectra. • Typical mass spectra of common analyte groups like pesticides, persistent organic pollutants, drugs; explosives; and of frequently occurring impurities. • Quantification using external and internal standards and standard addition methods. Determination of the limits of detection and quantitation. • Applications covering food, water, flavor and fragrance, metabolomics, forensic and material analysis. The Handbook of GC-MS: Fundamentals and Applications is an essential reference for the daily GC-MS practice and application of new methods. It serves as an excellent introduction for newcomers as well as an educational resource about this analytical technique. Analytical chemists, chromatographers, environmental chemists, food chemists, and pharmaceutical chemists will find it of high practical use. |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, PhD, HANS Analytical Solutions |
| Publication |
|---|
|
Wiley-VCH publisher, see: https://www.wiley.com/en-us/Handbook+of+GC-MS%3A+Fundamentals+and+Applications%2C+4th+Edition-p-9783527847624. Print version: ISBN: 978-3-527-84762-4, March 2025, 928 pages eBook: facilitates online index search for subjects and compounds, link to references, copy of tables for method developments and images for internal use. |
| Picture |
|---|
| Abstract |
|---|
| Keywords: micro-SPE, µSPE, QuEChERS, QuPPe, pesticide analysis, extract clean-up, automation, LC-MS, GC-MS, food, environment, veterinary drugs, filtration Micro solid phase extraction (µSPE) represents a significant leap forward in the evolution of sample preparation techniques, offering enhanced efficiency, precision and sustainability. In this interview, Hans-Joachim Hübschmann, a seasoned expert with decades of experience in food safety, environmental analysis and chromatography, shares his insights on the benefits of µSPE. Hans discusses the key motivation behind the miniaturization of SPE, the role µSPE plays in modern laboratories and its potential to revolutionize various analytical workflows. With a rich background in developing automated sample preparation solutions, Hans provides valuable perspectives on how µSPE can enhance both laboratory performance and environmental sustainability. |
| Files |
|---|
| Unlocking the Power of Miniaturization in SPE: Insights From an Expert |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, PhD, HANS Analytical Solutions |
| Publication |
|---|
| Technology Networks Interview (www.technologynetworks.com) "Transform Analytical Chemistry With Micro Solid Phase Extraction", published: January 17, 2025. |
2024
| Picture |
|---|
| Abstract |
|---|
| Keywords: QuEChERS, QuPPe, pesticide analysis, extract clean-up, automation, LC-MS, GC-MS, food, environment How advantageous would it be to use an automated sample preparation setup for food, beverage and environmental samples? For a sustainable and green sample preparation we want to save consumables, solvents, and for sure this also reduces expenses. And, its possible, save the investment for additional instrument. Micro-SPE is the solution today. SPE is well-known. A huge number of applications and all sorts of sorbent materials. There is the classical enrichment mode aka load-wash-elute process, and also the scavenging mode load-elute in use. Scaling down the size of SPE allows us to do SPE as a micromethod, which is then called micro-SPE, or in short using the micro sign µSPE. This µSPE approach is not new. It used since more than 10 years in commercial laboratories for pesticides and forensics applications. The current efforts for a greener analytical chemistry also made the large instrument manufacturer aware of this nice possibility to get on a greener footprint and provide a faster and more reliable sample prep solution for their customers. CTC Analytics in Switzerland developed a new µSPE cartridge that is available with the required sorbent materials for food, environmental or pharmaceutical analysis. |
| Files |
|---|
| The New PAL Micro-SPE Cartridge for Automated Pesticides Extraction and Clean-up — Micro-SPE for Green Analytical Chemistry |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, PhD, HANS Analytical Solutions |
| Publication |
|---|
| 15th European Pesticide Residue Workshop 16. – 20. September 2024 | Zürich, Switzerland, CTC Analytics AG vendor seminar |
| Picture |
|---|
| Abstract |
|---|
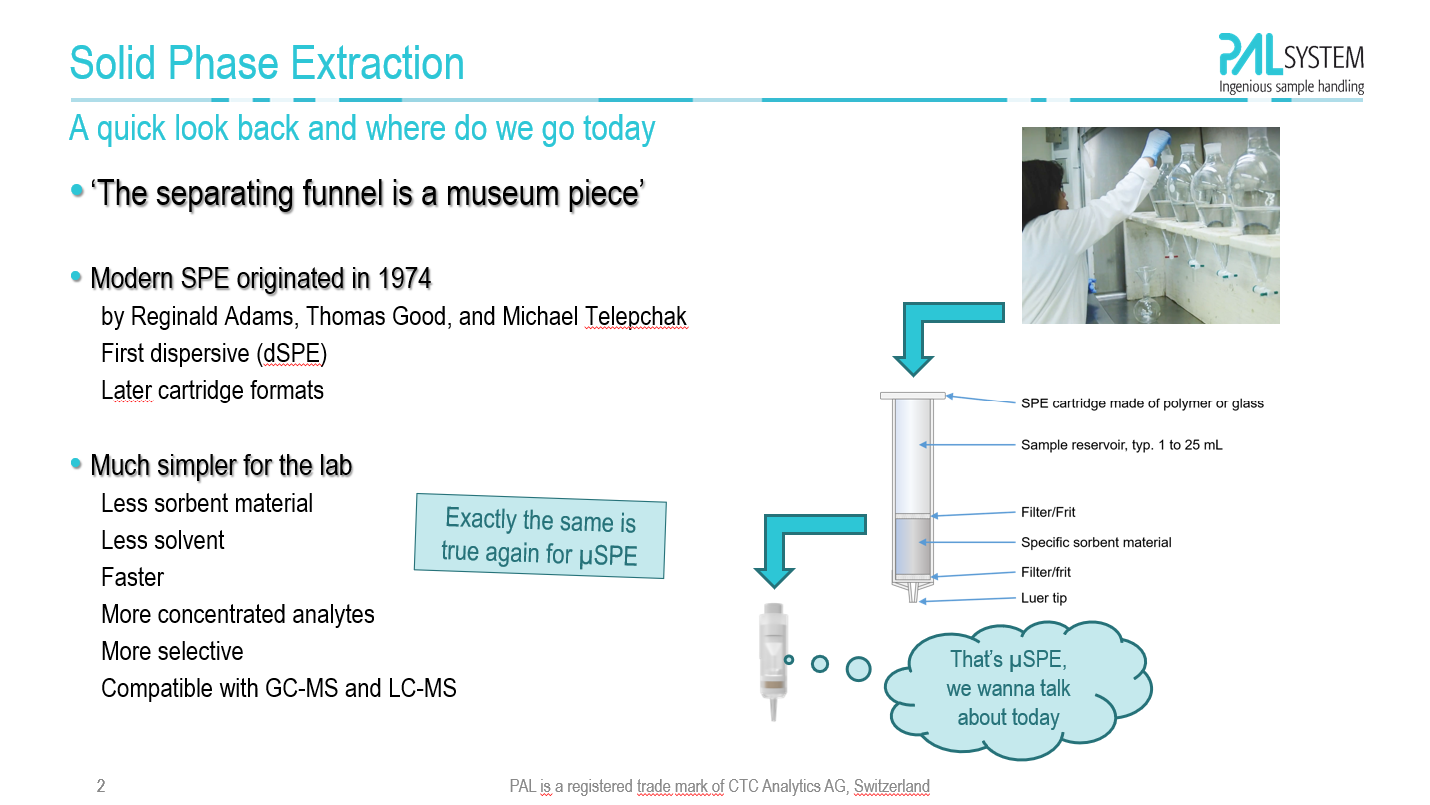
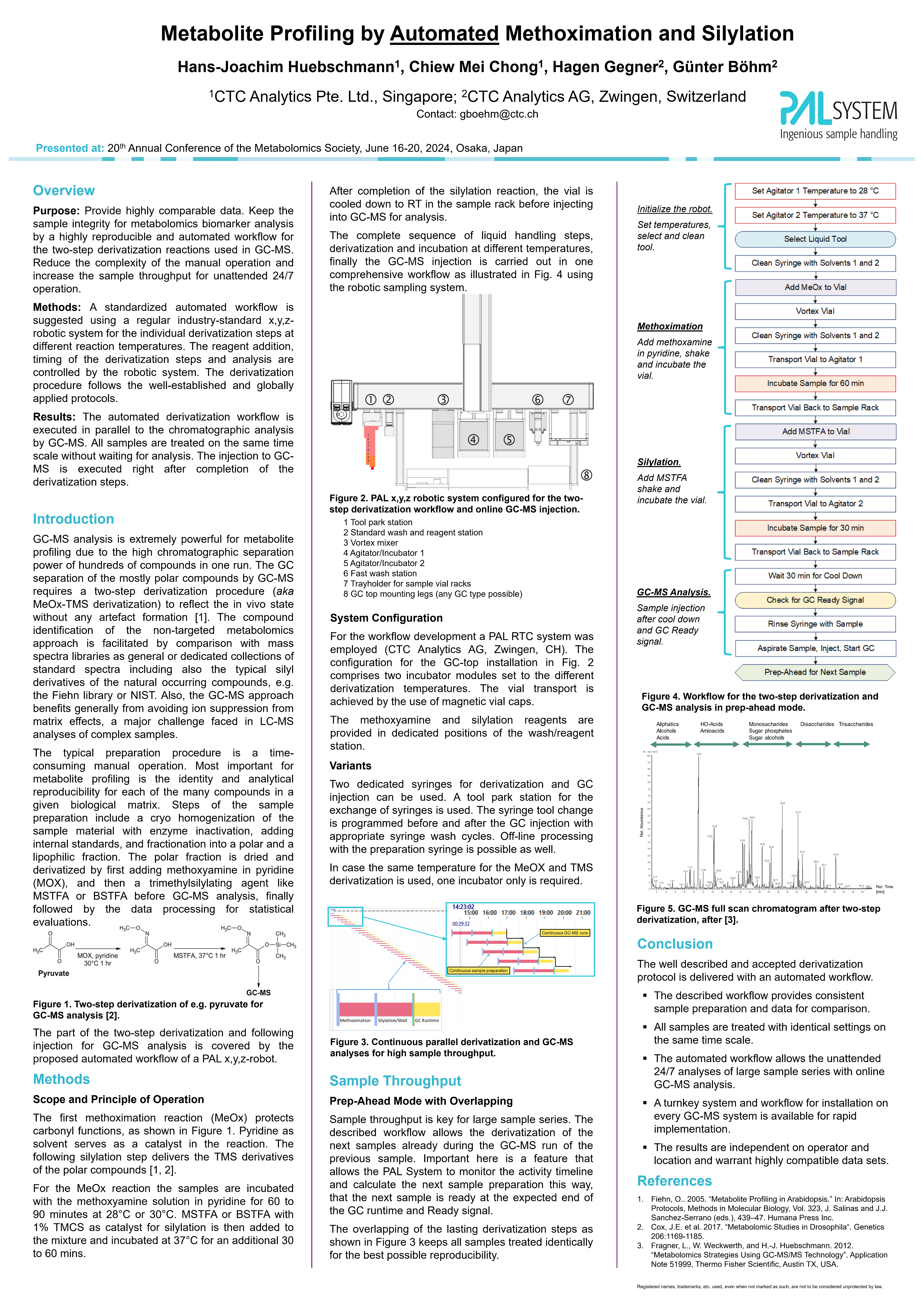
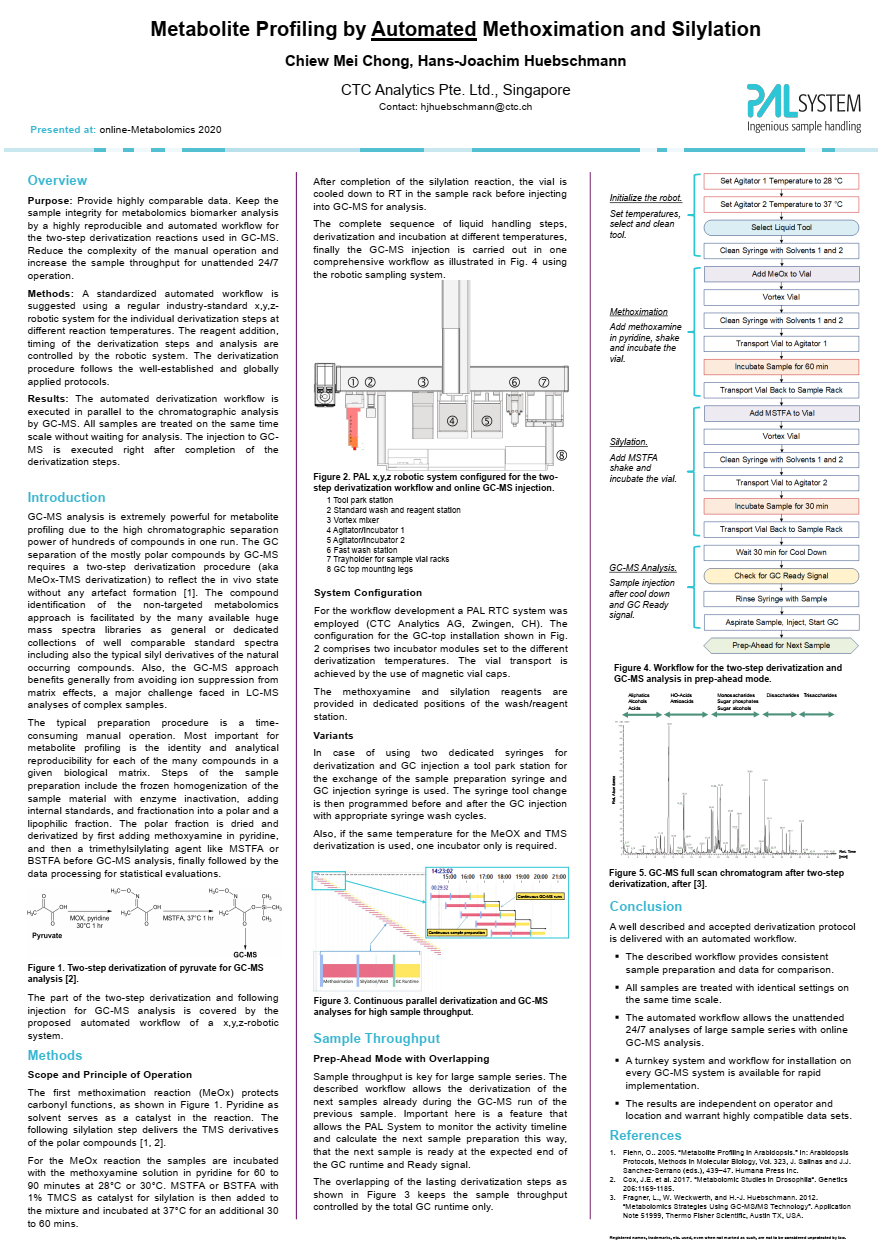
| Keywords: metabolomics, automation, derivatization, methoximation, silylation, GC-MSMS, identification, quantification, prep-ahead mode A standardized automated workflow is suggested using a regular industry-standard x,y,zrobotic system for the individual derivatization steps at different reaction temperatures (optional). The reagent addition, timing of the derivatization steps and analysis are controlled by the robotic system. The derivatization procedure follows the well-established and globally applied protocols. The automated derivatization workflow is executed in parallel to the chromatographic analysis by GC-MS. All samples are treated on the same time scale without waiting for analysis. The injection to GCMS is executed right after completion of the derivatization steps. |
| Files |
|---|
| Metabolite Profiling by Automated Methoximation and Silylation_Metabolomics v2 |
| Author(s) |
|---|
|
Hans-Joachim Huebschmann1, Chiew Mei Chong1, Hagen Gegner2, Günter Böhm2 1CTC Analytics Pte. Ltd., Singapore 2CTC Analytics AG, Zwingen, Switzerland |
| Publication |
|---|
| 20th Annual Conference of the Metabolomics Society, June 16-20, 2024, Osaka, Japan |
| Picture |
|---|
| Abstract |
|---|
| Keywords: MOSH, MOAH, online analysis, dual channel, LC-GC-FID, EFSA, Chrolibri Mineral oil hydrocarbon residues are found in numerous food or food contact materials originating from e.g. recycled card board, printing inks, or even the production machinery. The mineral oil hydrocarbons are classified as saturated (MOSH) and aromatic hydrocarbons (MOAH) with different toxicological context. MOAH compounds are considered genotoxic causing cancer. The EFSA confirmed 2023: “LOD as low as analytically possible”. Chromatographic separation of MOSH from MOAH can only be achieved by normal phase liquid chromatography (LC) collected as two separate fractions. Biogenic alkanes and olefins require prior online sample treatment to avoid overestimation for MOSH (AlOx clean-up) and even more serious for MOAH (per-acid epoxidation). Efficient sample analysis requires the transfer of the separated MOSH and MOAH LC fractions of approx. 450 µL for a parallel 2-channel GC separation while the LC column is backflushing. Quantitation has to be achieved for a wide range of chain length C10 to C50 by GC with FID detection (GC-FID). A dedicated data evaluation is required for the EFSA compatible reporting according to different C-chain length (Chrolibri). |
| Files |
|---|
| Fully Automated MOSH/MOAH Analysis |
| Author(s) |
|---|
|
Hans-Joachim Huebschmann, PhD, HANS Analytical Solutionsy Tobias Uber, PhD, Axel Semrau GmbH |
| Publication |
|---|
| 3rd European Sample Preparation Conference (EuSP2024) and the 2nd Green and Sustainable Analytical Chemistry Conference (GSAC2024), Chania, Greece |
| Picture |
|---|
| Abstract |
|---|
| Keywords: pesticides, extraction, µSPE clean-up, metabolomics, 2-step derivatization, reproducibility, SPME, automation, GC-MS/MS, PAL System This presentation covers two areas of analytical advances both benefiting from the PAL System automation of formerly cumbersome manual processes. For pesticides analysis the movement towards a Green Analytical Chemistry by further miniaturization of the sample preparation is the current hot topic. The well-know SPE moves on to micro-SPE (µSPE) with much less solvent and sorbent use. Automation by using µSPE cartridges makes it a fast and reliable workflow for high recoveries for pesticides in all kind of food matrices including fatty foods. Examples are presented from the leading reference laboratories. Automation is the big topic in metabolomics analysis as well. Large numbers of samples need to be processed for e.g. the identification of biomarkers for special properties. A very good reproducibility is key for meaningful comparison. The automated workflow of extraction and timely derivatization right before injection delivers the required analytical performance. Human and procedural bias is avoided. Examples demonstrate results for metabolomics analysis by 2-step derivatization of extracts by methoximation followed by silylation and GC-MS and GC-MS/MS analysis for rice varieties. The phenotype can be distinguished by a HS-SPME analysis showing distinct differences of these varieties. |
| Files |
|---|
| HJHuebschmann - Advances in Pesticide Analysis + Metabolomics 2-step derivatization |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, PhD, Consultant, HANS Analytical Solutions |
| Publication |
|---|
| Presentation July 2024 |
| Picture |
|---|
| Abstract |
|---|
| Keywords: Pesticide residues · Ethyl acetate extraction · Automated sample preparation · Micro-SPE extract clean-up · GCMS/MS Generic extraction methods for the multi-compound pesticide analysis of food have found their solid place in laboratories. Ethyl acetate and acetonitrile extraction methods have been developed as fast and easy to handle standard multi-compound methods, both feature benefits and limitations. The direct injection to gas chromatography can be impaired by a high burden of coextracted matrix, resulting in deterioration of the chromatographic system and matrix effects, requiring frequent maintenance. Therefore, common clean-up methods, such as dispersive solid-phase extraction, freeze-out of fats, or gel permeation chromatography, have been applied in clean-up. Automated clean-up using micro-solid-phase extraction (µSPE) is a recent development with several demonstrated advantages when employed in the analysis of pesticides and other contaminants in foods extracted with acetonitrile, but it has not yet been evaluated in this application using ethyl acetate for extraction. In this study, an automated procedure using µSPE cartridges was developed and established on an x,y,z robotic sampler for the raw extract clean-up and preparation of diluted samples for injection on a GC-MS/MS system. Validation experiments for 212 pesticides, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons in lettuce, avocado, raspberry, paprika, egg, and liver extracts were performed using µSPE with MgSO4, PSA, C18, and CarbonX. The performance in routine operation is briefly discussed. |
| Files |
|---|
| Schürmann Crüzer - 2024 - Automated uSPE clean‑up and GC-MSMS of pesticides in foods extracted with ethyl acetate |
| Author(s) |
|---|
|
Andreas Schürmann1 · Claudio Crüzer1 · Veronika Duss1 · Robin Kämpf1 · Thomi Preiswerk2 · Hans‑Joachim Huebschmann2 1 Cantonal Laboratory Zürich, Official Food Control Authority of the Canton of Zürich, Department Pesticide Analysis, Zurich, Switzerland 2 CTC Analytics AG, Zwingen, Switzerland. |
| Publication |
|---|
| Published online: 16 November 2023, ©The Author(s) 2023 |
2023
| Picture |
|---|
| Abstract |
|---|
|
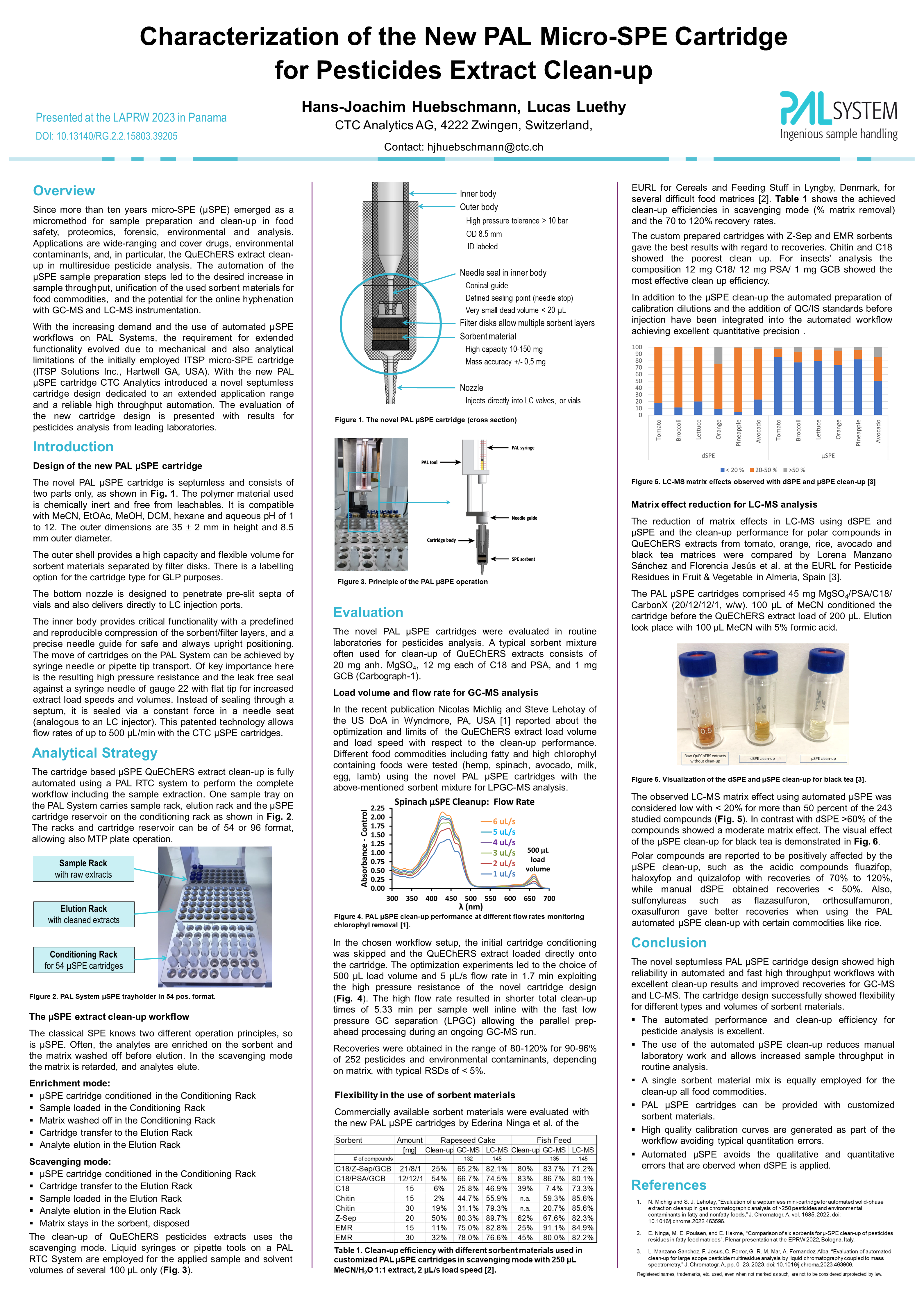
Micro-SPE (μSPE) emerged as an automated green micromethod for sample preparation and clean-up in food safety, proteomics, forensic, environmental and analysis since more than ten years. Applications are wide-ranging and cover drugs, environmental contaminants, and, in particular, the extract clean-up in multiresidue pesticide analysis. The automation of the μSPE sample preparation steps led to the desired standardization of the applied sorbent materials for extract clean-up for the large variety of food commodities, an increase in sample throughput, and the welcome potential for offline and online hyphenation with GC-MS, LC-MS or even IC analysis.
|
| Files |
|---|
| Analysis of Highly polar pesticides |
| Author(s) |
|---|
|
Florencia Jesús1, Amadeo Rodríguez Fernández-Alba1, Hans-Joachim Hübschmann2 1 Department of Chemistry and Physics, Agrifood Campus of International Excellence (ceiA3), University of Almeria, Spain 2 CTC Analytics AG, Zwingen, Switzerland. |
| Publication |
|---|
| Application Note published by CTC Analytics AG, Switzerland 12.2023 |
| Picture |
|---|
| Abstract |
|---|
| Keywords: Automated micro-solid-phase extraction, µSPE, pesticide analysis, QuEChERS, dispersive SPE Clean-up is essential during the multiresidue sample preparation process to remove undesired matrix components that may cause analytical interferences or suppression effect. However, its application generally by specific sorbents entails time-consuming work producing low recoveries for some compounds. Moreover, it usually needs to be adapted to the different co-extractives from the matrix present in the samples by using different chemical sorbents increasing the number of validation procedures. Therefore, the development of a more efficient and automated and unified clean-up procedure means a significant time reduction and laboratory work with improved performance. In this study, extracts from different matrices (tomato, orange, rice, avocado and black tea) were purified by manual dispersive clean-up (different procedures according to the matrix group) in parallel with an automated μSPE clean-up workflow, in both cases based on QuEChERS extraction. The latter procedure employed clean-up cartridges containing a mixture of sorbent materials (anhydrous MgSO4/PSA/C18/CarbonX) suitable for multiple matrices. All the samples were analysed by liquid chromatography mass spectrometry and the results obtained from both procedures have been compared in terms of the extract cleanness, performance, interferences, and sample workflow. At the levels studied, similar recoveries were achieved by both techniques (manual and automated) except for reactive compounds when PSA was used as the sorbent material producing low recoveries. However, the μSPE recoveries were between 70–120%. Furthermore, closer calibration line slopes were provided when μSPE was applied to the different matrix groups studied. It is important to note that up to 30% more samples per day can be analysed using an automated μSPE compared to the manual method (which requires shaking, centrifuging, then taking the supernatant and adding formic acid in ACN); it also provides good repeatability - an RSD (%) < 10%. Consequently, this technique is a very useful option for routine analyses, greatly simplifying the work of muti-residue methods. |
| Files |
|---|
| Manzano_Sanchez_Jesus_-_2023_-_Evaluation_of_automated_clean-up_for_large_scope_pesticide_multiresidue_analysis_by_LCMS https://doi.org/10.1016/j.chroma.2023.463906 |
| Author(s) |
|---|
| L. Manzano Sanchez, F. Jesus, C. Ferrer, M. M. Gomez-Ramos, and A. Fernandez-Alba, European Union Reference Laboratory for Pesticide Residues in Fruits & Vegetables. Agrifood Campus of International Excellence (ceiA3). Ctra. Sacramento S/N˚, University of Almeria, La Cañada de San Urbano, Almeria 04120, Spain. |
| Publication |
|---|
| L. Manzano Sanchez, F. Jesus, C. Ferrer, M. M. Gomez-Ramos, and A. Fernandez-Alba, “Evaluation of automated clean-up for large scope pesticide multiresidue analysis by liquid chromatography coupled to mass spectrometry,” J. Chromatogr. A, pp. 0–23, 2023, doi: 10.1016/j.chroma.2023.463906. Poster presentation at the 14th European Pesticide Residue Workshop (EPRW) 2022, Sep 19-23, 2022 in Bologna, Italy. |
2022
| Picture |
|---|
| Abstract |
|---|
| The goal of this development was to establish a generic clean-up procedure for the extracts of the ethylacetate extraction method which is useful for all incoming food types without requirement for different clean-up procedures dedicated to different food types. An automated extract cleanup was envisioned for an improved sample throughput on GCMSMS systems providing less manual variability and more reproducible pesticides recoveries for a wide variety of food commodities. The comparison of the automated µSPE workflow with an earlier manual dSPE method showed very good compliance within the normal and accepted error range in pesticide analysis for a wide range of food commodities with additional advantages for data quality and sample throughput. |
| Files |
|---|
| Preiswerk Huebschmann_uSPE_EtOAc_poster_final |
| Author(s) |
|---|
|
Thomi Preiswerk1, Hans-Joachim Huebschmann1, Claudio Cruetzer2, Veronika Duss2, Andreas Schuermann2 1CTC Analytics AG, Industriestrasse 20, 4222 Zwingen, Switzerland 2Cantonal Laboratory Zürich, Official Food Control Authority of the Canton of Zürich, Department Pesticide Analysis, Zürich, Switzerland |
| Publication |
|---|
| Poster presentation at the 14th European Pesticide Residue Workshop (EPRW) 2022, Sep 19-23, 2022 in Bologna, Italy |
| Picture |
|---|
| Abstract |
|---|
| Using PAL Robotic Tool Change (RTC) system, a fully automated QuEChERS was developed for the extraction and clean-up of organochlorine and organophosphate pesticides from homogeneous samples. The automated QuEChERS workflow includes extraction with acetonitrile, salting out with saturated sodium chloride solution and the clean-up with PAL µSPE prior to injection into the GC-MS/MS for analysis. Method validations were achieved by using the automated matrix matches calibration spiked from 1 ng/mL to 100 ng/mL of organochlorine and organophosphate pesticide standards into the PAL µSPE-cleaned tomato juice and red wine samples. The matrix match calibration curve linearities were at R2 0.995 or better. By spiking 10 ng/mL of pesticide standards into a raw tomato juice and red wine, the recoveries were obtained in the range of 70% - 130%, with n = 6 samples to determine the automated workflow precisions. |
| Files |
|---|
| Chong Huebschmann - Next Generation µSPE Cartridge for Automated Pesticide Analysis EPRW2022 Poster |
| Author(s) |
|---|
|
Chiew Mei Chong1, Hans-Joachim Huebschmann1, Thomi Preiswerk2 1CTC Analytics Asia Ple. Ltd., 117610 Singapore 2CTC Analytics AG, Zwingen, Switzerland. |
| Publication |
|---|
| Poster presentation at the 14th European Pesticide Residue Workshop (EPRW) 2022, Sep 19-23, 2022 in Bologna, Italy |
| Picture |
|---|
| Abstract |
|---|
| GC-MS analysis is extremely powerful for metabolite profiling due to the high chromatographic separation power, and the unique capability of compound identification by library search. The analysis of the mostly polar compounds by GC-MS requires a two-step derivatization procedure to reflect the in vivo state without any artefact formation. The methoximation reaction protects carbonyl functions. Pyridine serves as a catalyst in the reaction. The silylation step delivers trimethylsilyl derivatives of the polar compounds for GC-MS analysis. The methoximation reaction incubates the sample with the methoxyamine solution in pyridine for 60 to 90 minutes at 28°C. N-Methyl-N-(trimethylsilyl)trifluoracetamide or N,O-Bis(trimethylsilyl)trifluoroacetamide with 1% chlorotrimethylsilane as catalyst for silylation is then added to the mixture and incubated at 37°C for an additional 30 mins. After completion of the reaction, the vial is cooled down to room temperature in the sample rack before injection into GC-MS for analysis. The complete sequence of liquid handling steps, derivatization and incubation at different temperatures, including GC-MS injection is carried using a robotic sample handler. The poster presented describes in detail the automated workflow and system configuration of an x,y,z-robotic autosampler for online and offline two-step derivatization for GC-MS analyses. |
| Files |
|---|
| Boehm - 2022 - Metabolite Profiling by Automated Methoximation and Silylation_Metabolomics |
| Author(s) |
|---|
|
Guenter Boehm1, Chiew Mei Chong2, Hans-Joachim Huebschmann2 1 CTC Analytics AG, Zwingen, BL, Switzerland 2 CTC Analytics Pte. Ltd., Singapore |
| Publication |
|---|
| Presented at the Swiss Metabolomics Society Annual Meeting, September 29th 2022, Lausanne, Switzerland |
| Picture |
|---|
| Abstract |
|---|
| GC-MS is the standard analysis technique in food safety and environmental analysis. A huge number of samples is prepared in routine labs for regulated compounds and with standardized methods. Data of legal impact are critically scrutinized. The traditional manual sample preparation is known to be the root cause of error, doubt and severe throughput limitations. The proven concepts for automated workflows in food safety analysis for pesticides analysis using micro-SPE, for off-flavors mainly geosmin/3-MIB with SPME, and nitrosamine analysis by LLE from water are presented. |
| Files |
|---|
| Author(s) |
|---|
| Hans-Joachim Hübschmann, CTC Analytics Ltd. Pte., Singapore |
| Publication |
|---|
| Presentation at the Singapore Analytical Science Conference, July 4-8, 2022, web based.Presentation at the Singapore Analytical Science Conference, July 4-8, 2022, web based. |
| Picture |
|---|
| Abstract |
|---|
| The QuEChERSER mega-method has recently been introduced to quantify and identify a wide range of chemical residues (pesticides, veterinary drugs, environmental contaminants, among others) in nearly all types of foods. The approach calls for taking a small amount of the initial extract to cover analytes amenable to liquid chromatography, and the remainder is salted out for analysis by gas chromatography (GC), both with mass spectrometry (MS) based detection. In the case of GC-MS(/MS), the extract undergoes automated robotic mini-cartridge solid-phase extraction (SPE) cleanup in a technique known as μSPE or instrument-top sample preparation (ITSP). In 2022, a septumless mini-cartridge for μSPE was introduced to improve upon the ITSP design. The new design houses a bed of 20 mg anhydrous MgSO4, 12 mg each of C18 and primary secondary amine sorbents, and 1 mg of graphitized carbon black, the latter substituting for CarbonX used in the ITSP product. The septumless μSPE mini-cartridge employs a different gripping mechanism with the syringe needle that allows leak-free operation at higher flow rates (e.g. 10 μL/s), whereas the ITSP design is limited to 2 μL/s. Based on cleanup and analyte elution profiles, the extract load volume and flow rate was increased in μSPE for QuEChERSER from 300 μL at 2 μL/s to 500 μL at 5 μL/s, which improved accuracy of results, sped the cleanup step, and obviated the need for micro-vial inserts in the receiving vials. The new design also reduced both the amount and consistency of dead (void) volumes in the mini-cartridges from 83 ± 14 μL to 52 ± 7 μL for 200-600 μL load volumes. Normalization of peak areas to internal standards led to recoveries between 80 and 120% with typical RSDs <5% in low-pressure GC-MS/MS of 227-242 out of 252 pesticides, polychlorinated biphenyls, polybrominated diphenyl ethers, and polycyclic aromatic hydrocarbons in hemp powder, spinach, whole milk, egg, avocado, and lamb meat. |
| Files |
|---|
| Project: Evaluation of a septumless mini-cartridge for automated solid-phase extraction cleanup for GC-MS analysis of >250 pesticides Project: Evaluation of a septumless mini-cartridge for automated solid-phase extraction cleanup for GC-MS analysis of >250 pesticides |
| Author(s) |
|---|
|
Nicolás Michlig1,2, Steven J. Lehotay1 1US Department of Agriculture, Agricultural Research Service, Eastern Regional Research Center, 600 East Mermaid Lane, Wyndmoor, PA 19038, USA 2Facultad de Ingeniería Química, Programa de Investigación y Análisis de Residuos y Contaminantes Químicos (PRINARC), Universidad Nacional del Litoral, Santa Fe 3000, Argentina |
| Publication |
|---|
| N. Michlig and S. J. Lehotay, “Evaluation of a septumless mini-cartridge for automated solid-phase extraction cleanup in gas chromatographic analysis of >250 pesticides and environmental contaminants in fatty and nonfatty foods,” J. Chromatogr. A, vol. 1685, 2022, doi: 10.1016/j.chroma.2022.463596. |
| Picture |
|---|
| Abstract |
|---|
| Keywords: pesticide residue analysis, automation in residue analysis, micro-SPE, digitization, smart consumables Worldwide, food testing laboratories are confronted with large sample loads of fresh and processed commodities, and any delay in evaluating perishable items may undermine their shelf life and commercial value. Because of the growing awareness of food safety, supply chain stakeholders must strictly adhere to the applicable food legislation and obtain all necessary certifications to promote international trade. Newer tools for managing a safe food supply chain are therefore warranted. To comply with trade regulations, pesticide residue testing in foods is essential. Regulatory compliance analysis of multiclass pesticides requires numerous sample preparation steps and instrumental (GC-MS/MS and LC-MS/MS) runs that are labor-intensive and time-consuming. Over the past couple of decades, laboratory automation has evolved from moving samples to miniaturization through robotics. In simple terms, automation is the process of substituting human interactions with robotic interventions controlled by computer applications. Dilution, derivatization, pH adjustment, liquid extraction, mixing, centrifugation, and evaporation are all examples of potentially automated steps. While many laboratories lack automated systems due to their prohibitively high costs, automation in food testing has attracted widespread attention because of its ability to offer robust data in a timely manner. |
| Files |
|---|
| Automation in Pesticide Residue Analysis in Foods: A Step towards Smarter Laboratories and Green Chemistry Automation in Pesticide Residue Analysis in Foods: A Step towards Smarter Laboratories and Green Chemistry |
| Author(s) |
|---|
|
Kaushik Banerjee1 and Hans-Joachim Hübschmann2 1National Reference Laboratory, ICARNational Research Centre for Grapes, Pune 412307, India 2CTC Analytics Asia Pte. Ltd., Singapore 117610 |
| Publication |
|---|
| Kaushik Banerjee and Hans-Joachim Hübschmann, Automation in Pesticide Residue Analysis in Foods: A Step toward Smarter Laboratories and Green Chemistry, ACS Agric. Sci. Technol. 2022, 2, 3, 426–429. |
| Picture |
|---|
| Abstract |
|---|
| This Handbook of Automated Sample Preparation consists of two main parts. The first gives detailed information on the technical and analytical aspects of automation. A second part comprehensively covers real-world applications. Within those parts, sections address the integration of robotic sample preparation with GC-MS and LC-MS for online analysis. The appendix provides always necessary information for the implementation of workflows such as reference tables about needle gauges, solvent miscibility, solvent resistance, and discusses the tasks for preventive maintenance as well. A glossary of frequently used acronyms and terminologies concludes this practical addendum. With more than 600 citations of reference literature, a rich pool for access to related scientific publications for further reading is provided. The section on the analytical and technical aspects of automation focuses on the workflow concepts and the analytical functionality available with tools and modules used on x,y,z-robotic sample preparation systems. The operational principles are discussed with a detailed technical and analytical description, the intended application, and integration into workflows. The comprehensive section of automated solutions demonstrates proven experience. Such examples are ready for implementation in laboratories and allow individual customization. Commented step-by-step workflows are provided with typical results which can be the basis and starting point for individual standard operation procedures (SOPs). |
| Author(s) |
|---|
| Hans-Joachim Huebschmann |
| Publication |
|---|
| Hans-Joachim Huebschmann, "Automated Sample Preparation: Methods for GC-MS and LC-MS", 480 pages, Wiley-VCH, February 2022. Print ISBN: 978-3-527-34507-6 eBook ISBN: 978-3-527-81752-8 Automated Sample Preparation: Methods for GC-MS and LC-MS | Wiley |
2021
| Picture |
|---|
| Abstract |
|---|
| This presentation shows a newly developed micro-SPE (µSPE) method to automate the clean-up of PAHs from vegetable oil, here sunflower oil, using an industry standard x,y,z-robotic sampler. As the method is automated, labor is reduced. In addition, the applied µSPE cartridges need only 4 μL of sample and 360 μL acetonitrile for elution. A significant reduction of organic solvent compared to the manual method can be achieved, providing a true green analytical method. The presented method is the first automated µSPE method analyzing a pure sample. Furthermore, we show how a complex SPE method can be transferred to the µSPE concept [1-4]. The achieved µSPE method LOQ values exceed the regulations. We also discuss how sensitivity can be increased for future applications of screening for even lower PAH levels. |
| Files |
|---|
| Eyring Preiswerk - 2021 - Automated Clean-up PAH samples_EPRW poster final |
| Author(s) |
|---|
|
P. Eyring1, T. Preiswerk2, H.-J. Hübschmann3, H.L. Frandsen1, L. Duedahl-Olesen1 1Danish Technical University, Lyngby, Denmark 2CTC Analytics AG, Zwingen, Schweiz 3CTC Analytics Asia Pte. Ltd., Singapore |
| Publication |
|---|
| Poster presentation at the Latin America Pesticide Residue Workshop (LAPRW) 2021, May 18-20, 2021, online |
| Picture |
|---|
| Abstract |
|---|
| The preparation of working standards for calibration in analytical laboratories is a recurring, labor-intensive task. ▪ Manual liquid handling steps are often error prone and subject to individual variation. ▪ The SANTE analytical quality control document sets the requirements for the preparation of calibration standards: • Reference substances and stock solutions should be stored in a cool place, e. g. in a freezer, away from light and moisture. • Standards should be remixed after equilibration to room temperature. • Punctured septa should be replaced as soon as possible. • Standard vials should be permanently labelled. • The documentation should ensure full traceability of all steps. • Date of preparation, identity, mass and volume of the reference standard and the identity and volume of the solvents must be recorded. |
| Files |
|---|
| Breski Huebschmann - Poster Automated Preparation of Calibration Standards |
| Author(s) |
|---|
| T. Breski1, H.-J. Huebschmann2 1 Axel Semrau GmbH, Sprockhövel, Germany 2 CTC Analytics Pte. Ltd, Singapore |
| Publication |
|---|
| Poster presentation at the Latin America Pesticide Residue Workshop (LAPRW) 2021, May 18-20, 2021, online |
| Picture |
|---|
| Abstract |
|---|
| A fully automated QuEChERS is developed by using the PAL Robotic Tool Change (RTC) system in analyzing organophosphate pesticide residuals in orange juices. The automated workflows included the extraction with acetonitrile, salting out and using a µSPE cartridge for sample matrix cleanup prior to injection into the GC-MS/MS. The common method validation techniques such as pre spike and post spike were fully integrated into the PAL RTC automated workflows as well. With the complete automated method validation, the organophosphate based pesticides calibration linearities in orange juice from 1ng/mL – 100ng/mL were at least 0.995. By spiking 10ng/mL of pesticides into the orange juice samples, recoveries were obtained in the of range 70% - 115%, while the precision (%RSDs) from pre-spike (n=7) and post spikes (n=6) under the same concentration were mostly less than 10%. The calculated Method Detection Limits (MDLs) of all the monitoring pesticides were in the range of 1.8ng/mL – 4.1ng/mL which were well below the general Maximum Residual Limits (MRLs) of 10 ng/g. |
| Files |
|---|
| Chong Lim - 2021 - Fully Automated QuEChERS of Organge Juice_LAPRW 2021_poster |
| Author(s) |
|---|
| Chiew Mei Chong, Gwen Lim, Jianxia Lv, Benson Zhu, Hans-Joachim Huebschmann, CTC Analytics Asia Ple. Ltd., 117610 Singapore. |
| Publication |
|---|
| Poster presentation at the Latin America Pesticide Residue Workshop (LAPRW) 2021, May 18-20, 2021, onlinee |
| Picture |
|---|
| Abstract |
|---|
| The automated injection to gas chromatography systems (GC) is most probably the widest use of the so called “autosamplers” for quantitative GC and its hyphenation to mass spectrometry (GC-MS). While simple autosamplers do sequence controlled injections only, the usage of robotic sample preparation systems like the PAL Systems work in an integrated fashion with GC and GC-MS systems. High precision for quantitative analysis is provided with excellent quality by the PAL System for every modern GC. The PAL System offers two liquid injection modes referred to as “Normal” for best quantitative performance, and “Fast” for high boiling matrix samples. This technical note describes the operation of the most widely used “Normal” injection mode for best quantitative precision data on any GC model equipped with a hot split/splitless injector. |
| Files |
|---|
| Highly Precise GC Injection with the PAL System - Whitepaper |
| Author(s) |
|---|
| Gwen Lim Sin Yee, Hans-Joachim Hübschmann, CTC Analytics Pte. Ltd., Singapore |
| Publication |
|---|
| PAL System, GC Application Note, Highly Precise GC Injection with the PAL System, 2021, CTC Analytics AG |
| Picture |
|---|
| Abstract |
|---|
| A fully automated quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction and extract clean-up method for gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–mass spectrometry (LC–MS) analysis is presented using an industry standard robotic x,y,z-sampling system. This article analyzes organophosphate pesticides from orange juice. The automated workflow used in the study includes the extraction with acetonitrile and salting out and using a μSPE cartridge for matrix cleanup prior to injection into a gas chromatography–tandem mass spectrometry (GC–MS/MS) system. The method validation techniques, such as pre-spike and post-spike, were fully integrated into the automated workflow. Calibration linearities of the organophosphate pesticides in orange juice matrix range from 1 to 100 ng/mL with a precision achieved better than 0.995 for all compounds. By spiking 10 ng/mL of pesticides into the orange juice samples, recoveries were obtained in the range of 70–115%, while the precision from pre-spike (n = 7) and post-spike (n = 6) under the same concentration was less than 10% RSD. The calculated method detection limits (MDLs) of the monitored pesticides were in the range of 1.8–4.1 ng/mL, which are well below the regulated maximum residue limits (MRLs) of 10 ng/g for these pesticides. |
| Files |
|---|
| Chong Huebschmann - 2021 - Fully Automated QuEChERS Extraction and Cleanup |
| Author(s) |
|---|
| Chiew Mei Chong, Hans-Joachim Huebschmann |
| Publication |
|---|
| LCGC, Special Issues, Special Issues-04-01-2021, Volume 39, Issue s1, Pages: 12–16 |
2020
| Picture |
|---|
| Abstract |
|---|
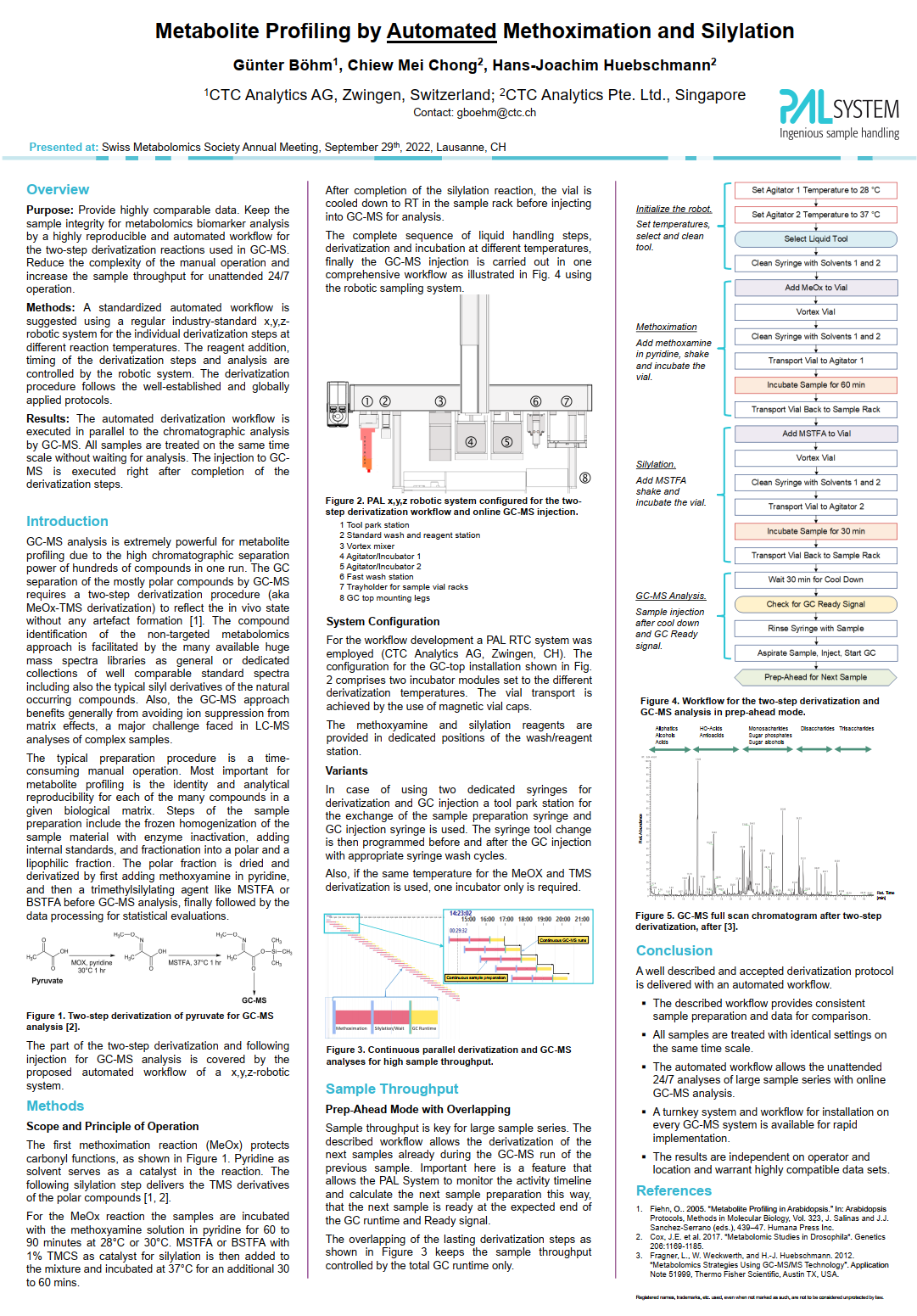
| Purpose: Provide highly comparable data. Keep the sample integrity for metabolomics biomarker analysis by a highly reproducible and automated workflow for the two-step derivatization reactions used in GC-MS. Reduce the complexity of the manual operation and increase the sample throughput for unattended 24/7 operation. Methods: A standardized automated workflow is suggested using a regular industry-standard x,y,zrobotic system for the individual derivatization steps at different reaction temperatures. The reagent addition, timing of the derivatization steps and analysis are controlled by the robotic system. The derivatization procedure follows the well-established and globally applied protocols. Results: The automated derivatization workflow is executed in parallel to the chromatographic analysis by GC-MS. All samples are treated on the same time scale without waiting for analysis. The injection to GCMS is executed right after completion of the derivatization steps. |
| Files |
|---|
| Metabolite Profiling by Automated Methoximation and Silylation Metabolomics |
| Author(s) |
|---|
| Chiew Mei Chong, Hans-Joachim Huebschmann |
| Publication |
|---|
| Poster at the online Metabolomics Conference 2020, CTC Analytics Pte. Ltd. https://doi.org/10.13140/RG.2.2.29085.03045 |
| Picture |
|---|
| Abstract |
|---|
| This study describes the effectiveness of the automated mini-SPE clean-up in multiresidue analysis of pesticides in a range of spice matrixes. The clean-up cartridge uses one standardized sorbent mix for all spices. The mini-SPE clean-up can be automated and executed in few minutes only in parallel to an ongoing GC-MS/MS analysis for improved productivity. The method facilitates highthroughput residue analysis in compliance with the regulatory requirements of sensitivity and method performance. |
| Files |
|---|
| Goon et al. - 2020 - Automated Clean-up and Analysis of Pesticides in Spices by Mini-SPE |
| Author(s) |
|---|
| Arnab Goon1,2, Raviraj Shinde1, Bappa Ghosh1, Kaushik Banerjee1 and Hans-Joachim Huebschmann3 1 National Referral Laboratory, ICAR-National Research Centre for Grapes, Pune, India 2 Thermo Fisher Scientific India Pvt. Ltd, Mumbai, India 3 CTC Analytics Asia Pte Ltd, Singapore |
| Publication |
|---|
| Poster at the 13th European Pesticide Residue Workshop (EPRW), May 11-15 2020 (web based) |
2019
| Picture |
|---|
| Abstract |
|---|
| This automated sample preparation workflow involved an X-Y-Z instrument autosampler, and a set of mini-SPE cartridges comprising cleanup sorbents. Spice samples were extracted by acetonitrile, and the extract was put into an autosampler vial for automated mini-SPE cleanup before analysis by GC tandem MS. For an efficient cleanup, three different sorbent compositions were compared along with various automated workflows. Results: For the relatively simple matrixes (e.g., coriander, cumin, and cardamom), the LOQ for the target pesticides was 10 ng/g with acceptable recovery, and precision. The method provided an LOQ of 10 ng/g for around 77% of the compounds in the relatively complex matrixes (e.g., turmeric, chili powder, and black pepper). The remainder of the compounds had satisfactory recoveries at 20 ng/g and higher levels. Conclusions: Given its time effectiveness and efficient analytical performance, this method can be adopted in commercial food testing laboratories for time-bound analysis of a large volume of samples. Highlights: The study describes effectiveness of the automated mini-SPE cleanup in multiresidue analysis of pesticides in a range of spice matrixes. The method facilitates high-throughput residue analysis in compliance with the regulatory requirements of sensitivity and method performance. |
| Files |
|---|
| Application of Automated Mini–Solid-Phase Extraction Cleanup for the Analysis of Pesticides in Complex Spice Matrixes by GC-MS/MS |
| Author(s) |
|---|
| Arnab Goon1,2, Raviraj Shinde1, Bappa Ghosh1, and Kaushik Banerjee1 1 National Referral Laboratory, ICAR-National Research Centre for Grapes, PO Manjri Farm, Pune 412307, India 2 Thermo Fisher Scientific India Pvt. Ltd, 403-404, Delphi “B” Wing, Mumbai 400076, India |
| Publication |
|---|
| A. Goon, R. Shinde, B. Ghosh, and K. Banerjee, “Application of Automated Mini–Solid-Phase Extraction Cleanup for the Analysis of Pesticides in Complex Spice Matrixes by GC-MS/MS,” J. AOAC Int., vol. 10, pp. 1–6, 2019. doi: 10.5740/jaoacint.19-0202. |
| Abstract |
|---|
| Since the first publication in 2003 the QuEChERS extraction method for pesticides in food (Anastassiades 2003) became a worldwide standard. While the extraction step is well standardized (AOAC 2007.1; CEN 15662), the clean-up of the resulting extracts from different matrices is still in active discussions. A well proven solution for a unified clean-up for all food matrices with only one cartridge type has been established with the use of µSPE cartridges (Hayward 2016). |
| Author(s) |
|---|
| Jianxia Lv, Hans-Joachim Huebschmann, Günter Boehm, and Reto Bolliger |
| Publication |
|---|
| Poster at the RAFA conference Prague, CTC Analytics AG, Zwingen, Switzerland.
Poster at the Separation Science Conference, 1./2. Nov 2017, CTC Analytics AG, Zwingen, Switzerland. |
| Picture |
|---|
| Abstract |
|---|
| Analyzing polycyclic aromatic hydrocarbons (PAHs) in drinking water becomes challenging especially for hard water (high mineral content water) and/or when sodium thiosulfate being added for treating chlorine content in drinking water during analysis. In this article, repeatability for determination of PAHs in drinking water using SPME Arrow (PDMS) immersion technique with gas chromatography mass spectrometer (GC/MS) was carried out. Single SPME Arrow had been used in this experiment to determine its durability. The repeatability (n=100) which characterized as %RSD was less than 10.93% for all analytes, ranged between 1.74 % - 10.93 %. The relative peak area of the 100th injection against the 1st injection peak area for all analytes ranged between 0.600 to 0.947; indicating SPME Arrow fiber is dutiful for at least 100 injections with immersion technique. In conclusion, fully automated SPME Arrow immersion technique with GC/MS analysis is an excellent option to analyze PAH compounds in drinking water; with higher throughput and robustness. |
| Files |
|---|
| Robustness of SPME Arrow Immersion Sampling Polycyclic Aromatic Hydrocarbons in Drinking Water |
| Author(s) |
|---|
| Gwen Yee Sin Lim, Thomi Preiswerk, Hans-Joachim Huebschmann, Chiew Mei Chong, Günter Böhm |
| Publication |
|---|
| Poster at the RAFA conference, CTC Analytics AG, Zwingen, Switzerland. |
2018
| Picture |
|---|
| Abstract |
|---|
| Among these studies, extractables and leachables represent a huge portion of the work the analyst does to characterize the substances potentially leaching into the product. The terms “extractables” and “leachables” are well defined by industrial working groups, 3, 4 mixed working groups5 (such as the Product Quality Research Institutione, the Leachables and Extractables Working Group, including pharmaceutical development scientists representing industry, health agencies, and academia) and subject experts. |
| Files |
|---|
| Gauriat et al. - Pharma Materials Study GC-MS Identification of Extractables and Leachables from Elastomer Material |
| Author(s) |
|---|
| Bénédicte Gauriat, Isabelle Froger, Damien Chevaillier, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific, Application Note 10398 |
2017
| Picture |
|---|
| Abstract |
|---|
| The extraction and clean-up of pesticides from food still raises particular challenges due to the high number and difference in chemical nature of the compounds embedded in food matrices of high complexity and highly varying compositions. Due to their polarity, molecular weight, stability, or ionization characteristics compounds are detected by GC and LC separation typically using MS/MS or accurate mass instrumentation (Raina 2011; Scherbaum 2008) In recent years the QuEChERS (Quick Easy Cheap Effective Rugged Safe) methodology (Anastassiades 2003) has been introduced in many food safety laboratories for pesticides. While the extraction step has been well standardized (AOAC 2007.1; CEN 15662) the clean-up of the resulting extracts still differs widely for different matrices and laboratories with the common goal to extend the GC, LC and MS maintenance cycles for increased productivity (Jiao 2016), and reducing matrix effects. The potential for high sample throughput using the QuEChERS extraction is impeded by laborious maintenance work (Hildmann 2014; Stahnke 2012). |
| Files |
|---|
| Automated micro-SPE Clean-up of QuEChERS Extracts for Multi-Residue Pesticide Analysis |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, Reto Bolliger, Guenter Boehm, Bruce D. Morris, Richard B. Schriner |
| Publication |
|---|
| Poster, Agilent Workshop Manchester, UK |
| Picture |
|---|
| Abstract |
|---|
| Keywords: Automated sample preparation, Bligh and Dyer, PAL RTC, SFC-MS, GC-MS Metabolomic analysis is prone to variation and errors due to manual processing at different states of sample preparation (e.g. extraction, purification, derivatization). Therefore, reproducible sample preparation is vital for ensuring reproducible and reliable results for large sample series. It is important to develop methods for reproducible, reliable and labor and time-saving sample preparation. The first step of modified Bligh and Dyer method, the addition of 1 mL cold methanol:chloroform:water solution and internal standards for both GC-MS and SFC-MS analysis, was performed manually, and subsequent procedures were performed by the automated protocol using a PAL RTC System. Aqueous layer and organic layer were collected in clean glass vials after the automated Bligh and Dyer method. The glass vials containing the aqueous layers were transferred to a centrifugal vacuum concentrator for drying samples manually. Then, vials were transferred to another PAL RTC system for the automated just-in-time derivatization and injection system for GC-MS analysis. The vials containing organic layers were transferred to auto-sampler of SFC-MS and subjected to target lipidomic analysis. The precision of relative peak area for each determined compound in the result of lipidomic and metabolomic analysis using automated protocol were equivalent to that of the manual protocol by a skilled technician. Additionally, these automated protocols facilitate overnight extraction procedure and compounds derivatization followed by on-line GC-MS analysis, resulting in significant labor-saving sample preparation. |
| Author(s) |
|---|
| Yuki Soma1, Toshiyuki Yamashita1, Masatomo Takahash1, Takatomo Kawamukai2, Teruhisa Shiota2, Kayoko Yamada2, Hans-Joachim Huebschmann3, Kuniyo Sugitate4, Takeshi Serino4, Hiromi Miyagawa5, Kenichi Suzuki5, Yoshihiro Izumi1, Takeshi Bamba1 1 Kyushu University, Fukuoka, Japan 2 AMR Inc., Meguro-ku, Japan 3 CTC Analytics AG, Zwingen, Switzerland 4 Agilent Technologies Co. Ltd, Hachioji, Japan 5 GL Sciences Inc., Shinjuku-ku, Japan |
| Publication |
|---|
| Poster at the Metabolomics Conference, Brisbane, Australia, July 26-29, 2017 |
| Picture |
|---|
| Abstract |
|---|
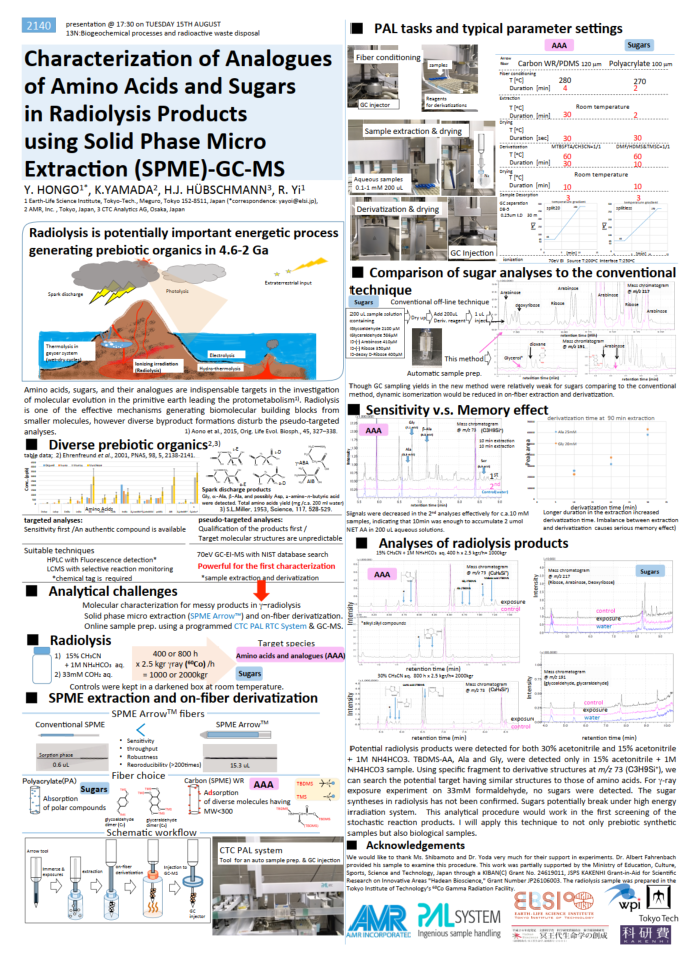
| Amino acids, sugars, and their analogues are indispensable targets in the investigation of molecular evolution in the primitive earth leading the protometabolism. Radiolysis is one of the effective mechanisms generating biomolecular building blocks from smaller molecules, however diverse byproduct formations disturb the pseudo-targeted analysis. Analysis by SPME extraction and on-fiber derivatization. |
| Files |
|---|
| Characterization of Analogues of Amino Acids and Sugars in Radiolysis Products using Solid Phase Micro Extraction |
| Author(s) |
|---|
| Yayoi Hongo, Kayoko Yamada, Hans-Joachim Huebschmann, R. Yi |
| Publication |
|---|
| Poster presentation at the Goldschmidt Conference 2017 |
| Picture |
|---|
| Abstract |
|---|
| 3-Monochloropropane-1,2-diol (3-MCPD), 2-monochloropropane-1,3-diol (2-MCPD) and glycidol known as contaminants in foodstuffs. MCPD fatty acids may occur during the refinement process of oils and fats at high temperature in the presence of chloride containing salts. Refinement is an essential chemical and physical process. Only through this kind of treatment undesired odorants, flavoring substances and possible traces of toxic compounds like pesticides, heavy metals or mycotoxins are eliminated. Increasing importance of the analysis of these contaminants due to their carcinogenicity. In March 2016, the European Food Safety Authority (EFSA) declared a reduced value for the tolerable daily uptake of 0.8 μg/kg body weight for 3-MCPD. |
| Files |
|---|
| DGF Fast & Clean - Auomated MCPD Analyses |
| Author(s) |
|---|
| Tobias Uber, Marco Nestola, Hans-Joachim Huebschmann, and Andreas Bruchmann |
| Publication |
|---|
| Poster at the Separation Science Conference, Singapore Nov 2-3, 2017 |
2015
| Picture |
|---|
| Description |
|---|
| The only comprehensive reference on this popular and rapidly developing technique provides a detailed overview, ranging from fundamentals to applications, including a section on the evaluation of GC-MS analyses. As such, it covers all aspects, including the theory and principles, as well as a broad range of real-life examples taken from laboratories in environmental, food, pharmaceutical and clinical analysis. It also features a glossary of approximately 300 terms and a substance index that facilitates finding a specific application. The first two editions were very well received, making this handbook a must-have in all analytical laboratories using GC-MS. |
| Files |
|---|
| Handbook of GC-MS 3rd ed. Forword by Kaushik_Banerjee Handbook of GC-MS 3rd ed. Introduction Handbook of GC-MS 3rd ed. Preface Handbook of GC-MS 3rd ed. Table of Contents |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| 3rd Edition 2015, Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 880 pages. Handbook of GC-MS: Fundamentals and Applications |
| Picture |
|---|
| Abstract |
|---|
| Analysis of a multi-residue mixture of pesticides at these trace levels is extremely challenging due to the complex extracts resulting from sample preparation. The instrument method also involves a number of mass transitions making the method even more complex. In order to obtain maximum sensitivity within minimum possible run time the instrument should be capable of detecting a large number of compounds in a single chromatographic run while maintaining superior sensitivity. Thermo Scientific™ TSQ™ 8000 Evo triple quadrupole GC-MS/MS system is an excellent tool in this regard. Enhanced velocity optics (EVO) driving EvoCell collision cell technology provides high SRM transition speeds, precision, and sensitivity for even the most complex methods involving several pesticides within a short run time. Other advantages include timed-SRM and AutoSRM for SRM. |
| Files |
|---|
| Analysis of Multiresidue Pesticides in Cardamom by GC-MSMS |
| Author(s) |
|---|
| Shridhar Gawade, Aarti Karkhanis, Soma Dasgupta, Manish Kumar, Sunil T Kumar, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific, Mumbai, India, Application Note 10469 |
| Picture |
|---|
| Abstract |
|---|
| Aloe vera (Aloe barbadensis Mill.) is a succulent plant species used in herbal medicine since the beginning of the first century A.D. Extracts from Aloe vera are widely used in the cosmetics and alternative medicine industries, having rejuvenating, healing, or soothing properties. Indian gooseberry (Amla, Phyllanthus emblica ) demonstrates in vitro antiviral, antimicrobial, and anticancer properties. All parts of the plant are used in various Ayurvedic/Unani medicines as herbal preparations, including the fruit, seed, leaves, root, bark and flowers. Aloe vera and Amla juices are also available in the market for direct consumption. However, there is increasing public concern about health risks from pesticide residues in food leading the strict regulation of maximum residue levels (MRL) and total dietary intake of pesticide residues in foodstuffs. International bodies, such as the Codex Alimentarius Commission and the European Union (EU), exert a major influence on food safety testing globally, with strict legislation in this area. According to Reg. 396/2005, Article 20 of the EU legislation no specific MRLs for juices are present which means that the MRL applied for juice is the corresponding MRL for the raw agricultural commodity. |
| Files |
|---|
| Multi-Residue Pesticide Analysis in Herbal Juices using GC-MSMS |
| Author(s) |
|---|
| Shridhar Gawade, Aarti Karkhanis, Soma Dasgupta, Sunil T Kumar, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific, Mumbai, India, Application Note 10464 |
| Picture |
|---|
| Abstract |
|---|
| Gas chromatography coupled with mass spectrometry (GC-MS) is particularly well suited to the study of low molecular-weight metabolites that can be made amenable to gas chromatography by chemical derivatization. However, while metabolite profiling by GC-MS in full scan mode reports identities of metabolites and their normalized relative intensities, the ultimate requirement for a quantitative description of the metabolite pool is absolute concentration determination. When studying a plant such as rice, targeted metabolites need to be accurately quantified for use as tools for functional genomics or as biomarkers to discriminate between different traits of diverse rice quality. |
| Author(s) |
|---|
| Jeremy P. Matthews, Silvia Gemme, Hans-Joachim Huebschmann, Cindy Llorente, Rosario Jimenez, Nese Sreenivasulu |
| Publication |
|---|
| Thermo Fisher Scientific (2015) Application Note AN10419 |
2014
| Abstract |
|---|
| “We called it, almost from the beginning, a “workhorse instrument.” It really, to this day, is that and more. With real advantages in scan speed, sensitivity, ease of use, a linear output, and workhorse capability it really caught on in a hurry.” Bob Finnigan 2001 |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| White Paper 10427, Thermo Fisher Scientific, Singapore |
| Abstract |
|---|
| Gel permeation chromatography (GPC) is based on the principle of molecular size separation and uses a porous column material. GPC is especially well suited to food extracts generated by the popular QuEChERS method1, where the matrix consists mostly of triglycerides (fat), pigments and other biopolymers, which can be separated from the desired small molecule pesticide compounds2,3. A powerful means of sample purification, gel purification has a wide range of applications internationally, such as the US EPA method 3640A4. China has adopted many standards, including GB/T 19650-20065, SN/T 0123-20106, SN/T 2149-20087, SN/T 2915-20118, pertaining to GPC as a purification method for the analysis of pesticide residues in various types of food. |
| Author(s) |
|---|
| Jinshui Che, Chongtian Yu, Lina Liang, and Hans-Joachim Huebschmann |
| Publication |
|---|
| Application Note AN10422, Thermo Fisher Scientific, Shanghai, China |
| Abstract |
|---|
| This is the first nationwide survey on nitrosamines in Korea by the sensitive electron impact ionization method. In the past, the main analytical stream of NDMA and their family compounds has been based on GC-CI-MS/MS. This proven method has been provided sensitive and reliable data. However, EI method can be applied with enough sensitivity due do the advanced ion focusing and timed selected reaction monitoring (timed SRM) technology. In this study, instrumental analysis for ionization method(EI,CI), sensitivity optimization etc. were tested. Sample preparation methods between commercialized SPE cartridges were validated. Finally, the optimized method was applied to the 76 treated samples from nationwide drinking water treatment plants. |
| Author(s) |
|---|
| Jaewon W. Choi, Y.D. Kim, J.M. Jung, and Hans-Joachim Huebschmann |
| Publication |
|---|
| ASMS conference poster #2402, Baltimore, MD, USA, 2014 |
| Abstract |
|---|
| The analysis of alkylphenol compounds in food and environmental matrices, illustrating the productivity and high-quality results from the GC-MS/MS system with automated selected reaction monitoring (SRM) development. European regulations create high demand for analytical screening for these compounds at low levels in both water and food. Additionally, nonylphenol and nonylphenol ethoxylate have been part of a U.S. Environmental Protection Agency (EPA) action plan. |
| Author(s) |
|---|
| Inge De Dobbeleer, Joachim Gummersbach, Hans-Joachim Huebschmann |
| Publication |
|---|
| Application Note, Thermo Fisher Scientific, Dreieich, Germany |
| Abstract |
|---|
| Wir nannten es von Anfang an ein „Arbeitspferd“. Das ist es wirklich, bis zum heutigen Tag, und darüber hinaus viel mehr. Mit Vorzügen in Scan-Geschwindigkeit, Empfindlichkeit, einfacher Bedienung, einem linearen Signal, und seinem Leistunsvermögen setzte es sich schnell durch. (Bob Finnigan, 2001) |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| GIT Labor Fachzeitschrift 3 (2014) 18-27 |
2013
| Abstract |
|---|
| For the analysis of the 15 polycyclic aromatic hydrocarbons (PAH) classified as priority from the Scientific Committee on Food (SCF) and benzo[c]fluorene assessed to be relevant by the Joint FAO/WHO Experts Committee on Food Additives (JECFA) in food, a sensitive analytical method is necessary. Methods: A Fast-GC/HRMS method with a runtime of only 25 minutes using a TR-50ms column (10m x 0.1mm x 0.1μm) was developed. Results: 113 representative samples of commercial smoked German meat products were tested. The median of benzo[a]pyrene content was 0.03 mg/kg and therefore greater than a factor of 100 below the maximum level of 5 mg/kg. The determined LODs and LOQs were in the range of 0.003 - 0.01 mg/kg and 0.009 – 0.03 mg/kg, respectively. |
| Author(s) |
|---|
| Wolfgang Jira, Fredi Schwägele, Hans-Joachim Huebschmann, Heinz Mehlmann |
| Publication |
|---|
| Poster at the ISPAC, Internat. Symposium on Polycyclic Aromatic Compounds, Oregon State University, Corvallis, 08.-12.09.2013, Oregon. https://docplayer.org/amp/18684524-Aus-der-arbeit-des-max-rubner-instituts-standort-kulmbach |
| Abstract |
|---|
| In the fully automated GC-EI-MS/MS, automated SRM mode provided 60% faster set up time and high sensitivity than the conventional GC-MS/MS method. The instrumental detection limit (IDL) of NDMA was 0.3 pg. IDL of other nitrosamines were 0.3-0.5 pg ranges. This indicated the low ng/L range LOQs for treated water samples with SPE sample concentration. The Auto SRM methodology is 60% faster than the conventional MRM set up. Points of SPE method : UCT cartridge, separated N2 with manual purging etc. In the real treated sample applications, NDEA was detected from all samples. Results of this study indicating the current EPA method 521 should be reassessed. |
| Author(s) |
|---|
| Y.D. Kim, J.W. Choi, J.M. Jung, and Hans-Joachim Huebschmann |
| Publication |
|---|
| APCE-KSFEA-APIA conference poster, Nov 2013, Korea |
2012
| Picture |
|---|
| Abstract |
|---|
| The EU Commission classified 22 amines as proven or suspected human carcinogens. “Azo dyes which, by reductive cleavage of one or more azo groups, may release one or more of these aromatic amines in detectable concentrations, i.e. above 30 ppm in the finished articles or in the dyed parts thereof … may not be used in textile and leather articles which may come into direct and prolonged contact with the human skin or oral cavity”. The EU Directive 2002/61/EC has banned the use of dangerous azo colorants, placing textiles and leather articles colored with such substances on the market, and requested the development of a validated analytical methodology for control. Since the azo dyes are one of the longest known synthetic dyes, simple and inexpensive in preparation, available easily in bulk and in great variety, and rarely cause acute symptoms, the textile manufactures can be persuaded to use them despite the regulations — if the strict and reliable analytical control is not imposed. |
| Author(s) |
|---|
| Adi Purwanto, Alex Chen, Kuok Shien, Hans-Joachim Huebschmann |
| Journal/Publication |
|---|
| Thermo Fisher Scientific (2012) Application Note 10329, Singapore. |
| Files |
|---|
| Detection, Identification, and Quantitation of Azo Dyes in Leather and Textiles by GC/MS |
| Abstract |
|---|
| Purpose: To demonstrate the ability of the Thermo Scientific TSQ Quantum XLS Series Triple Quadrupole GC-MS/MS to analyze the 209 PCB congeners listed in EPA Method 1668A at low levels with high precision and can be used for screening of PCBs even in difficult matrices. Methods: Retention times were determined in full scan mode and then a timed-SRM method was developed. Standards were analyzed at concentrations as low as 20 fg on-column, and precision was determined at 100 – 300 fg. A variety of matrices were spiked at low levels. Results: Individual compound %RSDs ranged from 5 to 32 analyzing 9 replicate samples with an average for all 209 congeners of 13%. Low level matrix spikes demonstrated the selectivity of the instrument. |
| Author(s) |
|---|
| David Steiniger, Dirk Krumwiede, Eric Phillips, Hans-Joachim Huebschmann, Paul Silcock |
| Journal/Publication |
|---|
| Poster, PittCon 2012, Thermo Fisher Scientific, Austin, Texas USA. |
| Abstract |
|---|
| This poster describes the analysis of a large number of pesticide components from herbal and tea products using GC-MS/MS. These types of dried plant products usually deliver high matrix loaded extracts from the sample preparation, and hence, pose a particular analytical challenge to the chromatographic and mass spectrometer system for a sensitive quantitation. High analyte selectivity and long term robustness are key for the high productivity pesticide monitoring in a routine trace analysis lab. |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, Joachim Gummersbach, Nicole Rueckert, Johann Kirchner, Elmar Häfner |
| Publication |
|---|
| Poster, Thermo Fisher Scientific, Dreieich, Germany. |
| Abstract |
|---|
| Over millions of years, organisms developed incredible ways to survive and interact with their often hostile environments. Different molecular levels of proteins and metabolites as well as their interactions, including gene expression, and underlying hierarchy of biochemistry and physiology can be summarized as the “genotype-phenotype relationship”.1 This link between genotype and phenotype is an issue of great interest and leads to the need for analyses at different molecular levels, the so called “omics” approaches: transcriptomics (gene expression), proteomics (protein translation) and metabolomics (metabolic network). |
| Author(s) |
|---|
| Fragner, L., W. Weckwerth, and H.-J. Huebschmann. |
| Publication |
|---|
| Thermo Fisher Scientific (2012) Application Note 51999, Austin TX, USA. https://static.thermoscientific.com/images/D00847~.pdf |
| Abstract |
|---|
| Pesticides are widely used in agriculture to protect crops and to improve efficiency of production. Consequently, governments, food producers and food retailers have the duty to ensure that any residues occurring in foods for human consumption are at or below Statutory Maximum Residue Levels (MRLs). Regulation EC 396/2005 adopted in the European Union sets MRLs for more than 500 dif- ferent pesticides in over 300 different food commodities. |
| Author(s) |
|---|
| Inge de Dobbeleer, Joachim Gummersbach, Hans-Joachim Huebschmann, Anton Mayer |
| Publication |
|---|
| LCGC - The Application Notebook (2012) September 1, 9. Thermo Fisher Scientific (2012) Application Note 52279, Dreieich, Germany |
2011
| Abstract |
|---|
| International regulations on maximum residue levels (MRLs) of pesticides in food cover hundreds of individual contaminants at the 10 ppb or below range. The analysis of citrus oil for pesticide contamination holds specific challenges. Pesticides that are used on citrus crops are concentrated along with the matrix in this oil. This matrix is known for its high boiling point components. The system of the Thermo Scientific TRACE GC Ultra and TSQ Quantum XLS GC–MS-MS allows for the analysis of more than 40 pesticides in citrus oil with minimal sample preparation. |
| Author(s) |
|---|
| Charles Lyle, Hans-Joachim Huebschmann, Eric Phillips |
| Publication |
|---|
| LCGC - The Application Notebook (2011) June 01, 2–5. |
| Abstract |
|---|
| The European Union Water Framework Directive seeks to lower detection and reporting limits for many contaminants (1). One class of these is organotins. Tributyltin compounds are considered the most hazardous, and several studies have shown the effect on shell malformation of oysters, imposex of marine snails and reduced resistance to infections (2,3). The method described uses the Thermo Scientific TSQ Quantum XLS triple quadrupole GC–MS–MS system to provide detection and quantification limits that go below regulatory requirements with the use of timed selected reaction monitoring (t- SRM). |
| Author(s) |
|---|
| Inge De Dobbeleer, Hans-Joachim Huebschmann, Anton Mayer, Joachim Gummersbach |
| Publication |
|---|
| LCGC - The Application Notebook (2011) August 02. |
| Abstract |
|---|
| The method described employs a triple quadrupole mass spectrometer equipped with hyperbolic quadrupole rods for increased selectivity to perform a trace level screening method for PCDD/Fs and PCBs and follows the well-established United States Environmental Protection Agency (US EPA) Method 1613A by using isotope dilution quantitation with 13C labelled internal standards. |
| Author(s) |
|---|
| Dirk Krumwiede, Hans-Joachim Huebschmann |
| Publication |
|---|
| LCGC North America 29 (2011) 2. |
2010
| Abstract |
|---|
| Because biomarker concentrations decrease with increasing thermal maturity, light oils contain only a low concentration of detectable biomarkers, and in consequence require instrumentation of highest selectivity and sensitivity. The information of source, thermal maturity, and secondary alteration processes are furthermore often revealed by only subtle variations in the isomeric distribution of such trace components. GC-MS/MS using the Thermo Scientific TSQ Quantum XLS is the premier analytical method for the sensitive characterization of “molecular fossil” biomarkers in crude oil. |
| Author(s) |
|---|
| Frank Theobald, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific (2010) Application Note 10261, Bremen, Germany |
| Abstract |
|---|
| Benzene and its alkyl derivatives toluene, ethylbenzene, and xylene (BTEX) have been classified by the World Health Organization (WHO) as a strong carcinogens causing cancer affecting human skin, lungs, and the nervous system. Quality control in the cigarette filter manufacturing process is a critical procedure for the tobacco industry. The reagents, accessories and raw filter material used in the production process may be a source of residual BTEX contamination, and hence require quality control. Therefore, the BTEX monitoring of cigarette filters in the tobacco industry has a significant impact on the choice and quality of filter materials. China is by far the largest tobacco producing country today, followed from a distance by Europe, North America and Latin America. In China, the directive YC/T 373-2010 for the control of propylene and C-fiber filter material requires the analysis of BTEX residue contaminations by gas chromatography-mass spectrometry (GC-MS).[3] The YC/T 373-2010 standard has high sensitivity requirements for the detection of BTEX. In this application note, we employ the method used in YC/T 373-2010 with the Thermo Scientific ISQ GC-MS for detection of BTEX compounds in propylene fiber cigarette filters. |
| Author(s) |
|---|
| Xuebin Zhang, Chongtian Yu, Lina Liang, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific, Shanghai, China, Application Note 10399 |
| Abstract |
|---|
| This method can be applied to fish and other fatty seafood samples to detect simultaneously the presence of aliphatic hydrocarbons and PAH contamination from crude oil found in the Gulf of Mexico. From the profile using GC-MS/MS, the method can be used to characterize the source of contamination. The method gives a quantitative indication as to whether levels of PAHs exceed safety limits for human consumption. |
| Author(s) |
|---|
| Klaus Mittendorf, Laszlo Hollosi, Ebru Ates, Katerina Bousova, Eric Phillips, Hans-Joachim Huebschmann, James Chang |
| Publication |
|---|
| Thermo Fisher Scientific, Food Safety Response Center (2010) Method 51991. |
| Abstract |
|---|
| Pesticides are widely used in agriculture to protect crops and to improve efficiency of production. Consequently, governments, food producers and food retailers have a duty to ensure that any residues occurring in foods for human consumption are at or below statutory maximum residue Ieveis (MRLs). Regulation EC 396/2005 adopted in the European Union sets MRLs for more than 500 different pesticides in over 300 different food commodities.1 Many of these MRLs are set at adefault value of 0.01 mg/kg, the typicallimit of determination of routine analytical methods. Thus, there is a requirement for residue laboratories to test a wide array of foods for a large number of pesticides at concentrations at or below 0.01 mg/kg , with low costs and fast turnaround. This is most often achieved using multiresidue methods based on the use of a combination of LC-MS- MS and GC-MS techniques to determine pesticide residues in a single generic solvent extract of the sample. One such example is the quick, easy, cheap, effective, rugged and safe (QuEChERS) procedure, which is based on acetonitrile extraction and dispersive solid-phase extraction. 2 Because acetonitrile is readily compatible with LC- MS- MS and allows analysis of several hundred pesticides this approach has gained in popularity. Many of the less polar semi-volatile pesticides not amenable to LC-MS can be analysed using GC- MS. Unfortunately, the analysis of acetonitrile QuEChERS extracts by GC-MS is more problematic, primarily due to degradation of the GC-column phase by the polar solvent, vapour overload of the insert Iiner due to the high thermal expansion coefficient, contamination of the system by co-extractives and low crop concentration in the final extracts. |
| Author(s) |
|---|
| Hetmanski, M. T., Fussell, R. J., Godula, M., Huebschmann, H.J. |
| Publication |
|---|
| The Applications Book, July/August 2010, 14-15. |
| Abstract |
|---|
| Pesticides are widely used in agriculture to protect crops and to improve efficiency of production. Consequently, governments, food producers and food retailers have a duty to ensure that any residues occurring in foods for human consumption are at or below Statutory Maximum Residue Levels (MRLs). Regulation EC 396/2005 adopted in the European Union sets MRLs for more than 500 different pesticides in over 300 different food commodities. 1 Many of these MRLs are set at a default value of 0.01 mg/kg, the typical limit of determination of routine analytical methods. Thus, there is a requirement for residue laboratories to test a wide array of foods for a large number of pesticides at concentrations at or below 0.01 mg/kg, with low costs and fast turnaround times (often< 48 hours). This is most often achieved using multi-residue methods based on the use of a combination of LC/MS/MS and GC/MS techniques to … |
| Author(s) |
|---|
| Michael T. Hetmanski, Richard Fussel, Michal Godula, Hans-Joachim Hübschmann |
| Publication |
|---|
| Thermo Fisher Scientific (2010) Application Note 51880, Bremen, Germany. |
2009
| Abstract |
|---|
| The physico-chemical diversity of biological small molecules makes a metabolomic analysis very difficult. Therefore, different analytical strategies are necessary and are ideally combined with each other. Analyses focusing on a group of metabolites or watching as many metabolites as possible at specified environmental or developmental stages is called metabolite profiling. On the other hand metabolite target analyses are restricted to specific metabolites of interest, which can be selectively monitored and quantified. There is a discrepancy of detectable peaks in a typical metabolomics sample and the number of assignable chemical identifications due to a restricted availability of non-complete reference libraries and technical problems such as ultracomplex coelution of compounds and non-optimal mass spectral deconvolution in gas chromatography-mass spectrometry. Therefore, we propose an integrative approach combining advantages of targeted analysis using multiple reaction monitoring (MRM) and full scan measurements to enhance the selectivity of the analysis, increase the number of identifiable and selectively quantifiable chemical structures and allow for absolute quantification strategies. |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, Lena Fragner, Wolfram Weckwerth, and Dwain Cardona |
| Publication |
|---|
| Poster, Thermo Fisher Scientific (2009) Austin TX, USA |
| Abstract |
|---|
| Die bisherigen Methoden zur Quantifizierung von Tetrahydrocannabinol-Carbonsäure in Urin arbeiten in der Regel nur bei relativ hohen Konzentrationen genau. Für Analysen im unteren Picogramm-oder gar Femtogrammbereich sind sie daher in der Regel ungeeignet. Wir stellen hier eine Methode vor, die durch hohe Wiederfindungsraten und eine hochsensitive Detektion charakterisiert ist und sich zur sicheren Identifizierung und Quantifizierung der THC-Carbonsäure in Harnproben auch von ProVolumeen mit geringer und unregelmäßiger Cannabis-Exposition eignet. |
| Author(s) |
|---|
| Hans-Ulrich Melchert, Hans-Joachim Hübschmann, Ellen Pabel |
| Publication |
|---|
| LaBo, Januar 2009 (German), 8-12. Also online at LaBo 01.01.2009: https://www.labo.de/analyseninstrumente/instrumentelle-analytik---fachbeitrag---analytik-der--thc-carbonsaeure.htm |
| Abstract |
|---|
| International regulations on the maximum residue levels of pesticides in food (MRLs) cover many hundreds of individual components at very low maximum residue limits in the range of only 10 ppb or even below [1]. More than 500 regulated pesticides can be analyzed by gas chromatography/mass spectrometry (GC/MS) while the group of highly polar pesticides is covered by liquid chromatography/mass spectrometry (LC/MS) methods [2]. GC/MS analysis of that many pesticide compounds at the MRL becomes an increasing challenge for food safety and governmental control strategies. This application describes the analytical methodology for a fast multiresidue pesticide determination using the unique highly selected reaction monitoring capabilities (H-SRM) and timed SRM data acquisition with the Thermo Scientific TSQ Quantum™ GC triple quadrupole mass spectrometer [3]. More than 1000 compounds can be screened in the multiple reaction monitoring method (MRM) in only one run for quantitation and confirmation of positive results. |
| Author(s) |
|---|
| Hans-Joachim Huebschmann; Weiguo Zhang, Lin Lu; Joachim Gummersbach |
| Publication |
|---|
| Poster at the 6th GCxGC Symposium, Portland OR, USA. Application Note AN10263 (2008) Thermo Fisher Scientific, Shanghai, China, and Bremen, Germany https://cdn.ymaws.com/www.casss.org/resource/resmgr/imported/ISCCE2009_FINALProgram_050809.pdf |
| Abstract |
|---|
| It is an excellent move that you look into this book! Analytical chemists want to be efficient and rapid: we are interested in a given task and the results should be available the next morning. This suggests taking the simplest route: “inject and see”, there is no time to fiddle about technology! The vendor of the possibly ex- pensive instrumentation might have highlighted the simplicity ofhis apparatus. This is a fundamental error. Efficient analysis presupposes a significant amount of time being devoted to understanding the method and the instrumentation. Not doing this in the beginning all too often exacts a high price at a later stage, e.g. in terms of a laborious and awkward method, endless troubleshooting and poor results. Knowledge of the technology is a prerequisite to make the best choices for a straight and simple method – from sample preparation to injection, chromatographic resolution and de- tection. If we are honest, we know that a staggering amount of our time is lost to trouble- shooting, and unless we have a deep insight into the technology, this troubleshooting is likely to be frustrating and ineffective (problems tend to recur). Hence investing time into understanding the technology is a wise investment for rapid (and reliable) analysis. …. It is great that an old hand in the field like Hans-Joachim Hübschmann took his time to bring the present knowledge into such a concise and readable form. Continue reading! |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| 2nd Completely revised and updated Ed., Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 719 pp. |
2008
| Abstract |
|---|
| For the analysis of the 15 polycyclic aromatic hydrocarbons (PAH) classified as priority from the Scientific Committee on Food (SCF) and benzo[c]fluorene (BcL) assessed to be relevant by the Joint FAO/WHO Experts Committee on Food Additives (JECFA) in food, a sensitive analytical method is necessary. In the past, many methods were established for only the analysis of the 16 priority PAH recommended by the US Environmental Protection Agency (16 EPA-PAH) but not for the 15 SCF-PAH and BcL. Therefore, at the Max-Rubner-Institut, Federal Research Institute of Nutrition and Food in Kulmbach, Germany, an analytical method with a runtime of 72 min was developed. To shorten this runtime without a loss of separation, a Fast-GC/high reso- lution MS (HRMS) method with a runtime of only 25 min using a TR-50ms column (10 m60.1 mm60.1 lm) was created. The repeatability (n = 3) of spiked PAH concen- trations in test-matrix tea ranged from 0.1 to 11%. The analytical parameters LOD (0.01–0.02 lg/kg) and LOQ (0.03–0.06 lg/kg) also in the test-matrix tea were deter- mined. A good linearity for all PAH (R2 A0.99) and averaged recoveries from 75 to 117% in the concentration range of1–20 lg/kg were achieved. |
| Author(s) |
|---|
| Katja Ziegenhals, Hans-Joachim Huebschmann, Karl Speer, and Wolfgang Jira |
| Publication |
|---|
| J. Sep. Sci. (2008). 1779–1786. https://doi.org/10.1002/jssc.200700641 |
| Abstract |
|---|
| International regulations on the maximum residue levels of pesticides in food (MRLs) cover hundreds of individual components at very low maximum residue limits – in the range of 10 ppb or lower. Currently, more than 300 regulated pesticides can be analyzed by gas chromatography/mass spectrometry (GC/MS) while a large group of highly polar pesticides is covered by liquid chromatography/mass spectrometry (LC/MS) methods. GC/MS analysis of that many pesticides at the MRL becomes an increasing challenge for quality and governmental control strategies. This application note provides the analytical methodology for a fast multi-residue pesticide determination using the unique capabilities of the Thermo Scientific TSQ Quantum™ GC triple quadrupole mass spectrometer for quantitation and confirmation of positive results. A particular challenge in the detection and quantification of a large number of pesticides in one run derives from the complex elution situation. Because many compounds partially or completely coelute, a high speed analyzer is required to generate a sufficient number of data points. This ensures reliable integration of overlapping chromatographic peaks. |
| Author(s) |
|---|
| Weiguo Zhang, Lin Lu, Hans-Joachim Huebschmann |
| Publication |
|---|
| LCGC Germany, 2008, May/June, 22–26. Thermo Fisher Scientific, Application Note AN10263 |
2007
| Abstract |
|---|
| The analysis of toxaphenes still is challenging, not limited to the almost impossible chromatographic separation of the vast amount of isomers, the injection technique as well as the ionization performance have a great influence over reproducibility, linearity and sensitivity. High resolution GC/MS offers the potential for a sensitive analysis providing the selectivity to overcome limiting interferences with matrix components as well as possible coelutions with other xenobiotica. Toxaphenes are included in the Annex A of the Stockholm Convention to protect human health and the environment from persistent organic pollutants (POPs). The international community has called for urgent global actions to reduce and eliminate releases of these listed chemicals including toxaphenes. Toxaphene is a complex mixture of polychlorinated camphenes (polychlorinated bornanes) that was first introduced in 1945 as broad band insecticide. The vast number and complexity of isomers results from the simple production process from the UV catalysed reaction of chlorine with camphene providing a product with a chlorine content of about 67 to 69%. The lipophilic, persistent and volatile nature of toxaphene has contributed to its global dispersion throughout freshwater and marine environments. Therefore, increased attention has been focused on toxaphene, both in the analytic and toxi- cologic fields. Toxaphene is believed to present a potential carcinogenic risk to humans. |
| Author(s) |
|---|
| Frank Theobald, and Hans-Joachim Huebschmann |
| Publication |
|---|
| Poster, Thermo Fisher Scientific (2007) Bremen, Germany |
| Abstract |
|---|
| Gas chromatographic (GC) methods require a specific chromatographic resolution for a given set of compounds in order to resolve analyte peaks from interferences, other analytes or homologues present in the same sample solution. This requirement is often a limitation when trying to shorten down GC run times in order to save valuable analysis time. A good example is the analysis of polychlorinated dioxins and furans (PCDD/Fs) by GC/mass spectrometry (GC/MS), e.g. in accordance to methods EPA 1613, or EN 1948. In order to achieve the requested GC resolution and other method requirements, typical total GC runtimes of up to 45 minutes, with the first peaks of interest elution after about 20 minutes. In other words, during the first 20 minutes no data acquisition takes place on the MS detector, wasting valuable instrument time. |
| Author(s) |
|---|
| Jens Griep-Raming, Stefan Ahlbrand, Dirk Krumwiede, and Hans-Joachim Huebschmann |
| Publication |
|---|
| Organohalogen Comp. 69 (2007) 1197–98 |
2006
| Abstract |
|---|
| Target compound analysis e.g. for polychlorinated dioxins, furans (PCDD/F), pesticides, persistent organic pollutants (POP) or pharmaceutical residues are typically performed by monitoring the compound specific ions at the expected retention time for each analyte. The DFS High Resolution GC/MS benefits from a unique technical feature referred to as the lock-mass technique for performing multiple ion detection (MID) analyses. The lock mass technique provides ease of use, combined with a maximum quantitative precision and certainty in analyte confirmation. This technical note describes the DFS mode of MID operation using a lock-plus-cali mass technique in detail and gives valuable hints for the setup of rugged routine methods. |
| Author(s) |
|---|
| Hans-Joachim Huebschmann, Jens Griep-Raming |
| Publication |
|---|
| Technical Note, Thermo Fisher Scientific, Bremen, Germany |
2005
| Abstract |
|---|
| 多氯联苯包括 209 种不同的单体 (同系物), 对健康有不同的影响. 虽然美国早在 1977 年就禁止生产 PCBs, 用 PCBs 制造的产品却仍然在使用. 由于多氯联苯在环境中降解缓慢, 所以它们可以被气流带到了地球的各个角落. PCBs 已经污染了地球上所有动物及人类的身体. 《 关于持久性有机污染物的斯德哥尔摩公约》 将 PCBs 列为世界上已知对人体和环境有害的十二种最危险的化合物之一. 尽管体内二恶英水平在缓慢稳定的下降, 反映出多种防止扩散的努力总的来说取得了一定效果, 然而 PCBs 污染水平在全球来讲预计不会发生变化 (2007 东京二恶英研讨会). 对二恶英水平的监测作为斯德哥尔摩公约的工作项目还将继续多年, 会涉及大量样品的采集, 尤其是对于危险的类二恶英 PCBs (dl-PCB) 来说. 值得一提的是共面 dl-PCB, 即非间位取代的 PCB, 由于具有与 2, 3, 7, 8-TCDD 类似的毒性, 成为食品安全控制的焦点. dl-PCBs 也对样品毒性当量 (TEQ) 值有显著贡献. |
| Author(s) |
|---|
| Dirk Krumwiede, Hans-Joachim Huebschmann |
| Publication |
|---|
| Thermo Fisher Scientific (2005) Application Note CM0105, Bremen, Germany |
2004
| Abstract |
|---|
| With the introduction of compound specific isotope analysis by isotope ratio monitoring GC/MS (irm-GC/MS) the immediate demand for similar applications using HPLC was created. In irm-GC/MS the carrier is helium which does not interfere with the essential combustion step prior to isotope ratio mass spectrometry (IRMS). In opposite the LC mobile phase has inhibited a similar direct conversion up to now. All earlier irm-LC/MS approaches were based on the removal of the liquid phase prior to combustion risking fractionation of the isotope ratios of the eluted compounds. To avoid such restrictions we developed a new continuous flow concept for the coupling of an HPLC system to the isotope ratio MS for d13C isotope ratio analysis. The differentiating idea of the new concept is to leave the compounds in the effluent and to combust them still in aqueous phase. Therefore this strategy is based on water and inorganic buffers as mobile phase. The resulting CO2 compound peaks are then separated from the effluent and analysed with the isotope ratio MS. |
| Author(s) |
|---|
| Dieter Juchelka, Hans-Joachim Huebschmann, Andreas Hilkert, Michael Krummen, Reinhold Pesch |
| Publication |
|---|
| Poster at the 33. Lebensmittelchemiker Tag in Bonn, Germany_Poster PA56, Thermo Electron Corporation, Bremen, Germany |
2001
| Author(s) |
|---|
| Hans-Joachim Huebschmann, Nigel Baldwin |
| Publication |
|---|
| Poster, Deutscher Lebensmittelchemikertag 2001 in Braunschweig, Germany, OmegaTech GmbH, Wendelsheim, Germany |
| Cite as: |
|---|
| Huebschmann, H.-J., & Baldwin, N. (2002). Entwicklung und Herstellung eines DHA-reichen Fermentationsöles aus Algen. Lebensmittelchemie, 56, 32 |
| Author(s) |
|---|
| Gerhard Kohn, Ruben Abril, Wulf Banzhaf, Hans-Joachim Hübschmann |
| Publication |
|---|
| Poster, Deutscher Lebensmittelchemikertag 2001 in Braunschweig, OmegaTech GmbH, Wendelsheim, Germany |
| Cite as: |
|---|
| Kohn, G., Abril, R., Banzhaf, W., & Huebschmann, H.-J. (2002). Neuartige Phospholipide bioaktiver Fettsäuren und Wege zu deren Herstellung. Lebensmittelchemie, 56, 33 |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| Weinheim: Wiley-VCH 2001, 592 pages, revised and supplemented English translation. |
1997
| Author(s) |
|---|
| Dirk Kohlmüller, Hans Joachim Hübschmann |
| Publication |
|---|
| in: O. Kaiser, R. E. Kaiser (Eds.): Chromatography - Celebrating Michael Tswett’s 125th Birthday, Düsseldorf: Incom Bureau 1997, 167 - 175 |
• Bestimmung von Phenoxyalkancarbonsäuren in Wasserproben durch automatische Target-Compound-Analyse
| Abstract |
|---|
| This article describes the development and application of the target compound analysis for phenoxyalkyl carbonic acid herbicides. The sample preparation takes advantage of an automated solid phase extraction System. The extracts are derivatised using diazomethane as reagent. The determination of the herbicides is tarried out with GC/MS in full scan mode. The full spectrum information allows the positive identification of the components prior to quantification with internal standard method. The automated method of sample preparation and compound determination is routinely used for high sample loads today. |
| Author(s) |
|---|
| Franz Kriegsmann, Hans Joachim Hübschmann |
| Publication |
|---|
| GIT Labor Fachz. 1 (1997), Sonderdruck |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| in: L. Matter (Ed.), Food and Environmental Analysis by Capillary Gas Chromatography, Heidelberg: Hüthig 1997, 119 - 171 |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| in: F. Pragst (Hrsg.): GTFCh-Symposium - Moderne Meßverfahren im Rahmen der Toxikologisch-Forensischen Begutachtung, Mosbach 18. - 19. April 1997, Verlag Helm: Heppenheim |
1996
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Weinheim: VCH Verlagsgesellschaft, 1996, 586 pages, 1st edition, Germany |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Fachbeitrag, LABO 5 (1996) |
1995
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| LABO 7/8 (1995) 42-49 |
| Abstract |
|---|
| Prinzipiell können in der Massenspektroskopie sowohl positive als auch negative Ionen erzeugt und analysiert werden. Die Elektronenstoß-lonisierung (electron impact, EI) als das Standardverfahren zur Ionenerzeugung liefert überwiegend positive Ionen. Die EI-Massenspektren haben ihre Bedeutung bei der Identifizierung und Bestätigung von Substanzen durch Bibliotheksvergleich. Die Ionisierungsenergie von 70 eV führt zu charakte- ristischen Fragmentierungen und Umlagerungsreaktionen der Moleküle, die eine wertvolle Information zur Strukturaufklärung darstellen. Durch die EI-Ionisierung ist die Massenspektroskopie zu einem universellen und massenspezifischen Detektionsverfahren in der GC/MS geworden. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| GIT Fachzeitschrift Labor, 39 (1995) 434-438 |
1994
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| in: L. Matter (Ed.): Lebensmittel- und Umweltanalytik mit der Kapillar-GC Weinheim: VCH Verlagsgesellschaft 1994, 117 - 172 |
| Abstract |
|---|
| A new method of mass spectrometric trace analysis using ion trap detectors based on positive chemical ionization with water as reactant is presented. The technique has advantages over the methods commonly used with regard to selectivity and sensitivity and facilitates detection of labile organic compounds. The specific increase in analytical response with water Cl for the analysis of nitrated aromatic substances is described. |
| Author(s) |
|---|
| Hans Joachim Hübschmann, Jörg Niebel, Andreas Landrock, H. Richter, H. Merten |
| Publication |
|---|
| Lecture on the Sixteenth International Symposium on Capillary Chromatography 1994, Riva, Italy |
1992
| Abstract |
|---|
| Die weite Verbreitung von Massenspektrometern als Detektoren in der Gaschromatographie hat die Diskussion über Bibliotheks- suchverfahren neu aufleben lassen. Insbesondere seit Einführung der Ion-Trap-Massenspektrometer im Jahre 1985 ist auch die ldentifizierung von Substanzen im Spurenbereich (pg-Bereich) einem breiten Anwenderkreis möglich geworden [I]. Be- dingt durch die unterschiedliche Softwareausstattung der Bench- top-GCMS-Systeme verschiedener Hersteller, haben sich, von der Anzahl der Installationen betrachtet, zwei Suchverfahren interna- tional durchgesetzt: INCOS und PBM, deren Leistungen und grundlegende Algorhythmen deshalb Gegenstand dieses Beitrages sind. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| LABO Analytica (1992) 102. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Part 1, LaborPraxis 10 (1990) 808 Part 2, LaborPraxis 12 (1990) 1014 |
1990
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT (1990) Application Report No. 75 |
| Abstract |
|---|
| Im ersten Teil des Beitrages wird die Funktion des Ion-Trap-Analysators erläutert und auf die Umsetzungsreaktionen bei der chemischen Ionisierung eingegangen. Ausführlich dargestellt werden die unterschiedlichen Betriebsweisen im EI- und Cl- Modus sowie die aus dem Einsatz der Cl-Ionisierung resultierenden Möglichkeiten für die massenspektrometrische Rückstandsanalytik auf Pflanzenschutzmittel. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| LaborPraxis (1990) Oktober, 808-812, and 1014-1017 |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT (1990) Application Report No. 75 |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| INCOM Sonderdruck (1990) 475 |
1989
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| INCOM Sonderdruck (1989) 485 |
1988
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT (1988) Application Report No. 68 |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT (1988) Application Report No. 67 |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT (1988) Application Report No. 63 |
1987
| Abstract |
|---|
| Nachfolgend soll das automatische Bestimmungsverfahren der Thermodesorption mit massenselektiver Detektion (Ion Trap Detektor ITD700) beschrieben werden. Dieses Verfahren bewährt sich besonder bei der Handhabung von komplizierten Matrices, wie sie z. B. bei Lösemitteln vorliegen. Sich überlagernde Komponenten können hier zweifelsfrei identifiziert und störungsfrei quantifiziert werden. Dies wird an zwei Beispielen, der Bestimmung von Lösemittelkomponenten und Narkosegasen ausgeführt. |
| Author(s) |
|---|
| Michael Tschickard, Hans Joachim Hübschmann |
| Publication |
|---|
| Finnigan MAT, Application Note No.67 (1987) Bremen, Germany |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| In: 2. Praktina, Europäisches Symposium fiir praktische instrumentelle Analytik, Krefeld, Hoppenstedt (1987) 77 |
| Abstract |
|---|
| Neben der Standardbetriebsweise durch ElektronenstoB-Ionisierung (EI) steht für den Ion Trap Detektor ITD 800 zur Absicherung von Strukturidentifizierungen und zur Durchführung von quantitativen Analysen hoher Selektivität jetzt auch die chemische Ionisierung (Cl) zur Verfügung. Aufgrund des einzigartigen Analysators sind zum Betrieb mit chemischer Ionisierung keinerlei Umbauten erforderlich. Die chemische Ionisierung ist vollständig von der Software kontrolliert und erlaubt dem Benutzer erstmals die Vorwahl der Reaktionszeiten. Der Einlaß des Reaktantgases erfolgt regelbar über ein Nadelventil direkt in die Meßzelle. Als Reaktantgas können alle herkömmlichen Cl-Gase wie Methan, iso-Butan, Ammoniak und sogar Wasser eingesetzt werden. Der Arbeitsbereich liegt bei Partialdrücken von ca. 10-5 Torr. Bei der chemischen Ionisierung wird das in den Analysator geleitete Reaktantgas durch einen kurzen Elektronen-Impuls ionisiert. Die hierbei gebildeten Reaktantionen werden gespeichert und reagieren anschließend mit den neutralen Probemoleküien, die durch Elution von der GC-Säule in den Analysator gelangen. Die gebildeten Substanz-Ionen werden gespeichert. ln dieser Phase ist der Elektronen-Impuls bereits abgeschaltet und die Ionenerzeugung erfolgt durch eine rein chemische Ionisierung. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Poster at the Symposium über klinisch toxikologische Analytik bei akuten Vergiftungen und Drogenmißbrauch, Salzburg 1987, Austria |
| Abstract |
|---|
| Heute ist in der organisch-chemischen Analytik der Nachweis und die Bestimmung einer wachsenden Anzahl von Stoffen in immer geringeren Konzentrationen erforderlich. In der Regel liegen die Konzentrationen der Analyten trotz aufwendiger Aufarbeitungsverfahren deutlich unterhalb einer begleitenden Matrix, dem chemischen Rauschen. Die Möglichkeiten der Anreicherung und des Cleanup weisen für diese Aufgabenstellungen bei Realproben Grenzen auf. Der bedeutende analytische Gewinn beim Einsatz von GC/MS-Systemen gegenüber einem Nachweis mit klassischen Detektoren wie ECD oder FID liegt in der Spezifität. Abgeleitet von der Zusammensetzung und Struktur der Analyten kann der Substanznachweis in der GC/MS mit zielgerichteter Selektivität für bestimmte Substanzklassen erfolgen, ohne daß die Universalität des „Detektors “eingeschränkt wird. Today, organic-chemical analysis requires the detection and determination of a growing number of substances in ever lower concentrations. As a rule, the concentrations of the analytes are well below a elaborate purification methods, the concentrations of the analytes are clearly below an accompanying matrix, the chemical noise. The possibilities of enrichment and cleanup have limits for these tasks with real samples. The significant analytical gain in the use of GC/MS systems compared to a detection with classical detectors such as ECD or FID lies in the specificity. Based on the composition and structure of the analytes, substance detection in GC/MS can be carried out with targeted selectivity for specific substance classes without limiting the universality of the detector" is restricted. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| GTFCh Media, 56-69. (German) |
| Abstract |
|---|
| Vor einem Jahr ist in der Bundesrepublik Deutschland die Verordnung über gefährliche Stoffe in Kraft getreten. Sie dient dem Schutz des Arbeitnehmers überall dort, wo gesundheitsgefährdende Stoffe eingesetzt werden. Ist das Auftreten gefährlicher Stoffe in der Luft am Arbeitsplatz nicht sicher auszuschließen, so muß geprüft werden, ob die maximalen Arbeitsplatzkonzentration (MAK-Werte) überschritten sind. Auch die Gesamtwirkung verschiedener gefährlicher Stoffe ist zu beurteilen. |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Chemische Rundschau 39 (1987) |
1986
| Abstract |
|---|
| Routinemäßig werden die Proben, nach entsprechender Aufarbeitung, meist mittels HPLC/Fluoreszenzdetektion oder GC/FID analysiert. Bei diesen Verfahren können sich besonders bei komplexen Matrizes Schwierigkeiten bei der Identifizierung und Quantifizierung der einzelnen Substanzen ergeben. Zur Absicherung solcher Ergebnisse ist eine GC/MS Analyse vorteilhaft. Bei Anwendung der Single Ion Technik wird meist auch eine höhere Empfindlichkeit erzielt. Durch die Vielfalt der bei einer GC/NS Analyse anfallenden Daten bieten sich eine Reihe von Möglichkeiten zur Auswertung der Chromatogramme an. Dabei kann vor allem durch die rechnerische Rekonstruktion der Ionenchromatogramme der für das Massenspektrum der gesuchten Verbindungen charakteristischen Ionen die Quantifizierung vereinfacht und verbessert werden. |
| Author(s) |
|---|
| Inge Trummer, P. Mohr, Andreas Wolf, Gerhard Miksche, Hans-Joachim Hübschmann and Hermann Katzlinger |
| Publication |
|---|
| Poster at the Deutsch-Österreichisches Chemikertreffen, Innsbruck, 12.-14. Mai 1986 |
| Abstract |
|---|
| The Finnigan MAT ION TRAP DETECTOR (ITD) is a sophisticated GC detector, which uses a new mass separation technology. It allows the chemist to separate analytical samples by mass as well as by retention time. Positive identification of the eluting compounds is achieved by supplementing GC retention time data with the mass spectra obtained with the ITD. This identification is further facilitated by the extensive library search capabilities of the system. The ITD is interfaced to any capillary GC by means of a flexible transfer line. It will accept the effluent from both narrow-bore (0.25 mm ID) and wide-bore (0.32 mm ID) fused silica columns. The transfer line includes an open split interface, which allows direct comparison of retention times produced with a conventional GC detector. Set-up of the ITD as well as data acquisition and processing are handled by an IBM Personal Computer XT. All acquired data and spectrum libraries (e.g. NBS library with more than 38.000 spectra) are stored on a 10 Mbyte hard disk. The application software programs are accessed via one letter commands. Those programs requiring additional input guide the user to respond with on-screen prompts. |
| Author(s) |
|---|
| Hans-Joachim Hübschmann, Ralf Schubert |
| Publication |
|---|
| Progress in Essential Oil Research (1986) 643 |
| Abstract |
|---|
| GC-MS Analyse von Ethylcarbamat in Obstbränden |
| Author(s) |
|---|
| Hans-Joachim Hübschmann, Lothar Matter |
| Publication |
|---|
| Chemische Rundschau 29. August (1986) 14 |
1985
| Abstract |
|---|
| ln den letzten Monaten wurde in vielen Weinen Diethylenglykol nachgewiesen. Die Bestimmung erfolgt meist gaschromategra- phisch auf Carbowax-Phasen und mit dem FID als Standard- Detektor. Die Identifizierung wird ausschließlich über die Retentionszeit erreicht. Hierbei wird die sichere Zuordnung des Diethy- lenglykoi-Peaks besonders im unteren ppm-Bereich erschwert durch die Fülle der Weininhaltsstoffe. Da zur Bewältigung des außerordentlich hohen Analysenanfalls der Wein in der Regel ohne Aufarbeitung direkt injiziert wird, ergibt sich, hauptsächlich durch hohen Zuckergehalt bedingt, eine starke Belastung des Injektors und der Trennsäule. ln der Folge können Retentionszeitverschiebungen des Diethylenglycols auftreten und damit zu Fehlinterpretationen führen. Die Quantifizierung des Diethylenglykols kann bei Verwendungen des FID durch koeluierende Inhaltsstoffe gestört werden. Es ist hierbei zu berücksichtigen, daß sowohl die Qualität als auch die Quantität der Weininhaltsstoffe denen eines Naturproduktes eigenen Schwankungen unterliegen. Unter den angegebenen Bedingungen eluiert Diethylenglycol (DEG) bei einer Retentionszeit von 5:18 min. Die Quantifizierung erfolgt durch Integration des Intensitätssignals auf den für DEG charakteristischen Massen m/z 75 bis m/z 76 auf Basis des zugehörigen Massenfragmentegramms. |
| Author(s) |
|---|
| Hans-Joachim Hübschmann, Hermann Katzlinger |
| Journal/Publication |
|---|
| Lebensmittel- und Biotechnologie 4 (1985) 148-149 |
| Abstract |
|---|
| Die Ringelektrode wird mit einer Gleichspannung und fiberlagertem Hochfrequenzfeld so betrieben, dab eine Speicherung von Ionen fiber den gesamten ausgew/ihlten Massenbereich erm6glicht wird. Nach der Ionisierungs-und Speicherphase wird das angelegte Feld ver/indert, so dab sukzessive definierte m/z-Werte instabil werden und den Speicherring in axialer Richtung verlassen. Die Ionen treten durch die Perforation der unteren Elektrodenkappe und treffen auf den Detektor auf. Das Detektorsignal korrespondiert als Funktion der Zeit mit dem Spektrum der zuvor gespeicherten Ionen. Das ITD kann fiber eine Transferline an jeden Gas-Chromatographen angeschlossen werden. Der Anschlug von Capillars/iulen erfolgt fiber eine offene Kopplung, die eine Beibehaltung |
| Author(s) |
|---|
| Hans Joachim Hübschmann |
| Publication |
|---|
| Fresenius' Journal/Publication für analytische Chemie 320, 7 (1985) 693-694. Cite this article: Hübschmann, H.J. Der Ion-Trap-Detektor: Technologie und Anwendungen. Z. Anal. Chem. 320, 693–694 (1985). https://doi.org/10.1007/BF00497162 |
(Use of Negative Chemical Ionisation Mass Spectrometry with Selective Reagent Gas Mixtures for the Detection of Diethylstilbestrol)
| Picture |
|---|
| Files |
|---|
| Hans Joachim Hübschmann - Thesis 1985 |
| Author(s) |
|---|
| Hans-Joachim Hübschmann |
| Publication |
|---|
| Thesis 1985 |
| Institution |
|---|
| Technical University, Institution for Food Chemistry, Berlin (Germany) |